Functionally Diverse Human T Cells Recognize Non-Microbial Antigens Presented By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigation of Functional Genes at Homologous Loci Identified Based
Journal of Atherosclerosis and Thrombosis Vol.22, No.5 455 Original Article Investigation of Functional Genes at Homologous Loci Identified Based on Genome-wide Association Studies of Blood Lipids via High-fat Diet Intervention in Rats using an in vivo Approach Koichi Akiyama, Yi-Qiang Liang, Masato Isono and Norihiro Kato Department of Gene Diagnostics and Therapeutics, Research Institute, National Center for Global Health and Medicine, Tokyo, Japan Aim: It is challenging to identify causal (or target) genes at individual loci detected using genome- wide association studies (GWAS). In order to follow up GWAS loci, we investigated functional genes at homologous loci identified using human lipid GWAS that responded to a high-fat, high-choles- terol diet (HFD) intervention in an animal model. Methods: The HFD intervention was carried out for four weeks in male rats of the spontaneously hypertensive rat strain. The liver and adipose tissues were subsequently excised for analyses of changes in the gene expression as compared to that observed in rats fed normal rat chow (n=8 per group). From 98 lipid-associated loci reported in previous GWAS, 280 genes with rat orthologs were initially selected as targets for the two-staged analysis involving screening with DNA microarray and validation with quantitative PCR (qPCR). Consequently, genes showing a differential expression due to HFD were examined for changes in the expression induced by atorvastatin, which was indepen- dently administered to the rats. Results: Using the HFD intervention in the rats, seven known (Abca1, Abcg5, Abcg8, Lpl, Nr1h3, Pcsk9 and Pltp) and three novel (Madd, Stac3 and Timd4) genes were identified as potential signifi- cant targets, with an additional list of 23 suggestive genes. -

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

Systems and Chemical Biology Approaches to Study Cell Function and Response to Toxins
Dissertation submitted to the Combined Faculties for the Natural Sciences and for Mathematics of the Ruperto-Carola University of Heidelberg, Germany for the degree of Doctor of Natural Sciences Presented by MSc. Yingying Jiang born in Shandong, China Oral-examination: Systems and chemical biology approaches to study cell function and response to toxins Referees: Prof. Dr. Rob Russell Prof. Dr. Stefan Wölfl CONTRIBUTIONS The chapter III of this thesis was submitted for publishing under the title “Drug mechanism predominates over toxicity mechanisms in drug induced gene expression” by Yingying Jiang, Tobias C. Fuchs, Kristina Erdeljan, Bojana Lazerevic, Philip Hewitt, Gordana Apic & Robert B. Russell. For chapter III, text phrases, selected tables, figures are based on this submitted manuscript that has been originally written by myself. i ABSTRACT Toxicity is one of the main causes of failure during drug discovery, and of withdrawal once drugs reached the market. Prediction of potential toxicities in the early stage of drug development has thus become of great interest to reduce such costly failures. Since toxicity results from chemical perturbation of biological systems, we combined biological and chemical strategies to help understand and ultimately predict drug toxicities. First, we proposed a systematic strategy to predict and understand the mechanistic interpretation of drug toxicities based on chemical fragments. Fragments frequently found in chemicals with certain toxicities were defined as structural alerts for use in prediction. Some of the predictions were supported with mechanistic interpretation by integrating fragment- chemical, chemical-protein, protein-protein interactions and gene expression data. Next, we systematically deciphered the mechanisms of drug actions and toxicities by analyzing the associations of drugs’ chemical features, biological features and their gene expression profiles from the TG-GATEs database. -
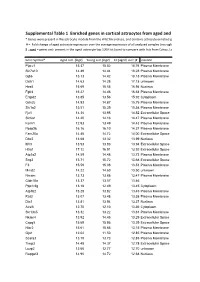
Supplemental Table 1 Enriched Genes in Cortical Astrocytes from Aged
Supplemental Table 1 Enriched genes in cortical astrocytes from aged and young-adult mice * Genes were present in the astrocyte module from the WGCNA analysis, and contains astrocyte enriched genes compared to microglia and oligodendrocytes # = Fold change of aged astrocyte expression over the average expression of all analyzed samples (microglia, astrocytes: young, old, with and without myelin contamination) $ ; aged = genes only present in the aged astrocyte top 1000 list (used to compare with lists from Cahoy, Lovatt, Doyle; see Fig. 4B), all = genes present in all astrocyte top 1000 lists Gene Symbol* Aged astr. (log2) Young astr.(log2) FC (aged/ aver.)# Location Ptprz1 15.37 15.02 18.76 Plasma Membrane Slc7a10 14.49 14.44 18.28 Plasma Membrane Gjb6 15.13 14.42 18.18 Plasma Membrane Dclk1 14.63 14.28 17.18 unknown Hes5 15.69 15.55 16.94 Nucleus Fgfr3 15.27 14.46 16.54 Plasma Membrane Entpd2 13.85 13.56 15.92 Cytoplasm Grin2c 14.93 14.87 15.75 Plasma Membrane Slc1a2 15.51 15.39 15.58 Plasma Membrane Fjx1 14.36 13.98 14.52 Extracellular Space Slc6a1 14.20 14.16 14.47 Plasma Membrane Kcnk1 12.93 13.49 14.43 Plasma Membrane Ppap2b 16.16 16.10 14.37 Plasma Membrane Fam20a 14.48 14.72 14.00 Extracellular Space Dbx2 13.68 13.32 13.99 Nucleus Itih3 13.93 13.93 13.94 Extracellular Space Htra1 17.12 16.91 13.92 Extracellular Space Atp1a2 14.59 14.48 13.73 Plasma Membrane Scg3 15.71 15.72 13.68 Extracellular Space F3 15.59 15.08 13.51 Plasma Membrane Mmd2 14.22 14.60 13.50 unknown Nrcam 13.73 13.88 13.47 Plasma Membrane Cldn10a 13.37 13.57 13.46 -

Recombinant T-Cell Immunoglobulin and Mucin Domain Containing Protein 4 (TIMD4)
RPH854Hu01 100µg Recombinant T-Cell Immunoglobulin And Mucin Domain Containing Protein 4 (TIMD4) Organism Species: Homo sapiens (Human) Instruction manual FOR RESEARCH USE ONLY NOT FOR USE IN CLINICAL DIAGNOSTIC PROCEDURES 12th Edition (Revised in Aug, 2016) 1 / 4 [ PROPERTIES ] Source: Prokaryotic expression Host: E.coli Residues: Glu25~Gln314 Tags: N-terminal His Tag Subcellular Location: Secreted Purity: > 95% Traits: Freeze-dried powder Buffer formulation: 100mMNaHCO3, 500mMNaCl, pH8.3, containing 0.01% SKL, 5% Trehalose. Original Concentration: 100µg/mL Applications: Positive Control; Immunogen; SDS-PAGE; WB. (May be suitable for use in other assays to be determined by the end user.) Predicted isoelectric point: Predicted Molecular Mass: 35.0kDa Accurate Molecular Mass: 40kDa as determined by SDS-PAGE reducing conditions. Phenomenon explanation: The possible reasons that the actual band size differs from the predicted are as follows: 1.Splice variants: Alternative splicing may create different sized proteins from the same gene. 2. Relative charge: The composition of amino acids may affects the charge of the protein. 3. Post-translational modification: Phosphorylation, glycosylation, methylation etc. 4. Post-translation cleavage: Many proteins are synthesized as pro-proteins, and then cleaved to give the active form. 5. Polymerization of the target protein: Dimerization, multimerization etc. [ USAGE ] Reconstitute in 100mM NaHCO3, 500mM NaCl (pH8.3) to a concentration of 0.1-1.0 mg/mL. Do not vortex. 2 / 4 [ STORAGE AND STABILITY ] Storage: Avoid repeated freeze/thaw cycles. Store at 2-8ºC for one month. Aliquot and store at -80ºC for 12 months. Stability Test: The thermal stability is described by the loss rate. -

The Title of the Dissertation
UNIVERSITY OF CALIFORNIA SAN DIEGO Novel network-based integrated analyses of multi-omics data reveal new insights into CD8+ T cell differentiation and mouse embryogenesis A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Bioinformatics and Systems Biology by Kai Zhang Committee in charge: Professor Wei Wang, Chair Professor Pavel Arkadjevich Pevzner, Co-Chair Professor Vineet Bafna Professor Cornelis Murre Professor Bing Ren 2018 Copyright Kai Zhang, 2018 All rights reserved. The dissertation of Kai Zhang is approved, and it is accept- able in quality and form for publication on microfilm and electronically: Co-Chair Chair University of California San Diego 2018 iii EPIGRAPH The only true wisdom is in knowing you know nothing. —Socrates iv TABLE OF CONTENTS Signature Page ....................................... iii Epigraph ........................................... iv Table of Contents ...................................... v List of Figures ........................................ viii List of Tables ........................................ ix Acknowledgements ..................................... x Vita ............................................. xi Abstract of the Dissertation ................................. xii Chapter 1 General introduction ............................ 1 1.1 The applications of graph theory in bioinformatics ......... 1 1.2 Leveraging graphs to conduct integrated analyses .......... 4 1.3 References .............................. 6 Chapter 2 Systematic -

Nuclear Organization and the Epigenetic Landscape of the Mus Musculus X-Chromosome Alicia Liu University of Connecticut - Storrs, [email protected]
University of Connecticut OpenCommons@UConn Doctoral Dissertations University of Connecticut Graduate School 8-9-2019 Nuclear Organization and the Epigenetic Landscape of the Mus musculus X-Chromosome Alicia Liu University of Connecticut - Storrs, [email protected] Follow this and additional works at: https://opencommons.uconn.edu/dissertations Recommended Citation Liu, Alicia, "Nuclear Organization and the Epigenetic Landscape of the Mus musculus X-Chromosome" (2019). Doctoral Dissertations. 2273. https://opencommons.uconn.edu/dissertations/2273 Nuclear Organization and the Epigenetic Landscape of the Mus musculus X-Chromosome Alicia J. Liu, Ph.D. University of Connecticut, 2019 ABSTRACT X-linked imprinted genes have been hypothesized to contribute parent-of-origin influences on social cognition. A cluster of imprinted genes Xlr3b, Xlr4b, and Xlr4c, implicated in cognitive defects, are maternally expressed and paternally silent in the murine brain. These genes defy classic mechanisms of autosomal imprinting, suggesting a novel method of imprinted gene regulation. Using Xlr3b and Xlr4c as bait, this study uses 4C-Seq on neonatal whole brain of a 39,XO mouse model, to provide the first in-depth analysis of chromatin dynamics surrounding an imprinted locus on the X-chromosome. Significant differences in long-range contacts exist be- tween XM and XP monosomic samples. In addition, XM interaction profiles contact a greater number of genes linked to cognitive impairment, abnormality of the nervous system, and abnormality of higher mental function. This is not a pattern that is unique to the imprinted Xlr3/4 locus. Additional Alicia J. Liu - University of Connecticut - 2019 4C-Seq experiments show that other genes on the X-chromosome, implicated in intellectual disability and/or ASD, also produce more maternal contacts to other X-linked genes linked to cognitive impairment. -

Environmental Influences on Endothelial Gene Expression
ENDOTHELIAL CELL GENE EXPRESSION John Matthew Jeff Herbert Supervisors: Prof. Roy Bicknell and Dr. Victoria Heath PhD thesis University of Birmingham August 2012 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Tumour angiogenesis is a vital process in the pathology of tumour development and metastasis. Targeting markers of tumour endothelium provide a means of targeted destruction of a tumours oxygen and nutrient supply via destruction of tumour vasculature, which in turn ultimately leads to beneficial consequences to patients. Although current anti -angiogenic and vascular targeting strategies help patients, more potently in combination with chemo therapy, there is still a need for more tumour endothelial marker discoveries as current treatments have cardiovascular and other side effects. For the first time, the analyses of in-vivo biotinylation of an embryonic system is performed to obtain putative vascular targets. Also for the first time, deep sequencing is applied to freshly isolated tumour and normal endothelial cells from lung, colon and bladder tissues for the identification of pan-vascular-targets. Integration of the proteomic, deep sequencing, public cDNA libraries and microarrays, delivers 5,892 putative vascular targets to the science community. -

The Mutational Landscape of Myeloid Leukaemia in Down Syndrome
cancers Review The Mutational Landscape of Myeloid Leukaemia in Down Syndrome Carini Picardi Morais de Castro 1, Maria Cadefau 1,2 and Sergi Cuartero 1,2,* 1 Josep Carreras Leukaemia Research Institute (IJC), Campus Can Ruti, 08916 Badalona, Spain; [email protected] (C.P.M.d.C); [email protected] (M.C.) 2 Germans Trias i Pujol Research Institute (IGTP), Campus Can Ruti, 08916 Badalona, Spain * Correspondence: [email protected] Simple Summary: Leukaemia occurs when specific mutations promote aberrant transcriptional and proliferation programs, which drive uncontrolled cell division and inhibit the cell’s capacity to differentiate. In this review, we summarize the most frequent genetic lesions found in myeloid leukaemia of Down syndrome, a rare paediatric leukaemia specific to individuals with trisomy 21. The evolution of this disease follows a well-defined sequence of events and represents a unique model to understand how the ordered acquisition of mutations drives malignancy. Abstract: Children with Down syndrome (DS) are particularly prone to haematopoietic disorders. Paediatric myeloid malignancies in DS occur at an unusually high frequency and generally follow a well-defined stepwise clinical evolution. First, the acquisition of mutations in the GATA1 transcription factor gives rise to a transient myeloproliferative disorder (TMD) in DS newborns. While this condition spontaneously resolves in most cases, some clones can acquire additional mutations, which trigger myeloid leukaemia of Down syndrome (ML-DS). These secondary mutations are predominantly found in chromatin and epigenetic regulators—such as cohesin, CTCF or EZH2—and Citation: de Castro, C.P.M.; Cadefau, in signalling mediators of the JAK/STAT and RAS pathways. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Steroid-Dependent Regulation of the Oviduct: a Cross-Species Transcriptomal Analysis
University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2015 Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis Katheryn L. Cerny University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Cerny, Katheryn L., "Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis" (2015). Theses and Dissertations--Animal and Food Sciences. 49. https://uknowledge.uky.edu/animalsci_etds/49 This Doctoral Dissertation is brought to you for free and open access by the Animal and Food Sciences at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Animal and Food Sciences by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

Serum Albumin OS=Homo Sapiens
Protein Name Cluster of Glial fibrillary acidic protein OS=Homo sapiens GN=GFAP PE=1 SV=1 (P14136) Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 Cluster of Isoform 3 of Plectin OS=Homo sapiens GN=PLEC (Q15149-3) Cluster of Hemoglobin subunit beta OS=Homo sapiens GN=HBB PE=1 SV=2 (P68871) Vimentin OS=Homo sapiens GN=VIM PE=1 SV=4 Cluster of Tubulin beta-3 chain OS=Homo sapiens GN=TUBB3 PE=1 SV=2 (Q13509) Cluster of Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 (P60709) Cluster of Tubulin alpha-1B chain OS=Homo sapiens GN=TUBA1B PE=1 SV=1 (P68363) Cluster of Isoform 2 of Spectrin alpha chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTAN1 (Q13813-2) Hemoglobin subunit alpha OS=Homo sapiens GN=HBA1 PE=1 SV=2 Cluster of Spectrin beta chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTBN1 PE=1 SV=2 (Q01082) Cluster of Pyruvate kinase isozymes M1/M2 OS=Homo sapiens GN=PKM PE=1 SV=4 (P14618) Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 Clathrin heavy chain 1 OS=Homo sapiens GN=CLTC PE=1 SV=5 Filamin-A OS=Homo sapiens GN=FLNA PE=1 SV=4 Cytoplasmic dynein 1 heavy chain 1 OS=Homo sapiens GN=DYNC1H1 PE=1 SV=5 Cluster of ATPase, Na+/K+ transporting, alpha 2 (+) polypeptide OS=Homo sapiens GN=ATP1A2 PE=3 SV=1 (B1AKY9) Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 Dihydropyrimidinase-related protein 2 OS=Homo sapiens GN=DPYSL2 PE=1 SV=1 Cluster of Alpha-actinin-1 OS=Homo sapiens GN=ACTN1 PE=1 SV=2 (P12814) 60 kDa heat shock protein, mitochondrial OS=Homo