713 by FERDINAND ACCORDING
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multivalent Metals and Polyatomic Ions 1
Name Date Comprehension Section 4.2 Use with textbook pages 189–193. Multivalent metals and polyatomic ions 1. Define the following terms: (a) ionic compound (b) multivalent metal (c) polyatomic ion 2. Write the formulae and names of the compounds with the following combination of ions. The first row is completed to help guide you. Positive ion Negative ion Formula Compound name (a) Pb2+ O2– PbO lead(II) oxide (b) Sb4+ S2– (c) TlCl (d) tin(II) fluoride (e) Mo2S3 (f) Rh4+ Br– (g) copper(I) telluride (h) NbI5 (i) Pd2+ Cl– 3. Write the chemical formula for each of the following compounds. (a) manganese(II) chloride (f) vanadium(V) oxide (b) chromium(III) sulphide (g) rhenium(VII) arsenide (c) titanium(IV) oxide (h) platinum(IV) nitride (d) uranium(VI) fluoride (i) nickel(II) cyanide (e) nickel(II) sulphide (j) bismuth(V) phosphide 68 MHR • Section 4.2 Names and Formulas of Compounds © 2008 McGraw-Hill Ryerson Limited 0056_080_BCSci10_U2CH04_098461.in6856_080_BCSci10_U2CH04_098461.in68 6688 PDF Pass 77/11/08/11/08 55:25:38:25:38 PPMM Name Date Comprehension Section 4.2 4. Write the formulae for the compounds formed from the following ions. Then name the compounds. Ions Formula Compound name + – (a) K NO3 KNO3 potassium nitrate 2+ 2– (b) Ca CO3 + – (c) Li HSO4 2+ 2– (d) Mg SO3 2+ – (e) Sr CH3COO + 2– (f) NH4 Cr2O7 + – (g) Na MnO4 + – (h) Ag ClO3 (i) Cs+ OH– 2+ 2– (j) Ba CrO4 5. Write the chemical formula for each of the following compounds. (a) barium bisulphate (f) calcium phosphate (b) sodium chlorate (g) aluminum sulphate (c) potassium chromate (h) cadmium carbonate (d) calcium cyanide (i) silver nitrite (e) potassium hydroxide (j) ammonium hydrogen carbonate © 2008 McGraw-Hill Ryerson Limited Section 4.2 Names and Formulas of Compounds • MHR 69 0056_080_BCSci10_U2CH04_098461.in6956_080_BCSci10_U2CH04_098461.in69 6699 PDF Pass77/11/08/11/08 55:25:39:25:39 PPMM Name Date Comprehension Section 4.2 Use with textbook pages 186–196. -

UV Spectroscopic Determination of the Chlorine Monoxide (Clo)/Chlorine Peroxide (Cloocl) Thermal Equilibrium Constant
Atmos. Chem. Phys., 19, 6205–6215, 2019 https://doi.org/10.5194/acp-19-6205-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. UV spectroscopic determination of the chlorine monoxide (ClO) = chlorine peroxide (ClOOCl) thermal equilibrium constant J. Eric Klobas1,2 and David M. Wilmouth1,2 1Harvard John A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA 2Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA Correspondence: J. Eric Klobas ([email protected]) Received: 22 October 2018 – Discussion started: 29 October 2018 Revised: 19 April 2019 – Accepted: 24 April 2019 – Published: 10 May 2019 Abstract. The thermal equilibrium constant between the net V 2O3 ! 3O2 (R5) chlorine monoxide radical (ClO) and its dimer, chlorine peroxide (ClOOCl), was determined as a function of tem- Within this cycle, the equilibrium governing the partitioning perature between 228 and 301 K in a discharge flow ap- of ClO and ClOOCl in Reaction (R1) is defined as follows. paratus using broadband UV absorption spectroscopy. A [ClOOCl] third-law fit of the equilibrium values determined from Keq D (1) [ClO]2 the experimental data provides the expression Keq D 2:16 × 10−27e.8527±35 K=T / cm3 molecule−1 (1σ uncertainty). A This thermal equilibrium is a key parameter that deter- second-law analysis of the data is in good agreement. From mines the nighttime partitioning of active chlorine in the the slope of the van’t Hoff plot in the third-law analy- winter–spring polar vortex. -

Implementation Guidelines for the Environmental Emergency Regulations 2011
Implementation Guidelines for the Environmental Emergency Regulations 2011 Print version Cat. No. En14-56/1-2011E ISBN: 978-1-100-19745-6 PDF version Cat. No. En14-56/1-2011E-PDF ISBN: 978-1-100-19746-3 Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: • Exercise due diligence in ensuring the accuracy of the materials reproduced; • Indicate both the complete title of the materials reproduced, as well as the author organization; and • Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the Government of Canada’s copyright administrator, Public Works and Government Services of Canada (PWGSC). For more information, please contact PWGSC at 613-996-6886 or at [email protected]. © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2011 Aussi disponible en français TABLE OF CONTENTS 1.0 PURPOSE OF THE IMPLEMENTATION GUIDELINES ................................................................................... 1 2.0 ENVIRONMENTAL EMERGENCY AUTHORITIES UNDER PART 8 OF CEPA 1999 ....................................... 3 3.0 BENEFITS -

Stratospheric Ozone Is Destroyed by Reactions Involving
20 Questions: 2010 Update Section II: THE OZONE DEPLETION PROCESS What are the chlorine and bromine reactions that destroy Q9 stratospheric ozone? Reactive gases containing chlorine and bromine destroy stratospheric ozone in “catalytic” cycles made up of two or more separate reactions. As a result, a single chlorine or bromine atom can destroy many thousands of ozone molecules before it leaves the stratosphere. In this way, a small amount of reactive chlorine or bromine has a large impact on the ozone layer. A special situation develops in polar regions in the late winter/early spring season where large enhancements in the abun- dance of the most reactive gas, chlorine monoxide, leads to severe ozone depletion. tratospheric ozone is destroyed by reactions involving before it happens to react with another gas, breaking the cata- Sreactive halogen gases, which are produced in the chemi- lytic cycle, and up to tens of thousands of ozone molecules cal conversion of halogen source gases (see Figure Q8-1). The during the total time of its stay in the stratosphere. most reactive of these gases are chlorine monoxide (ClO), bro- Polar Cycles 2 and 3. The abundance of ClO is greatly mine monoxide (BrO), and chlorine and bromine atoms (Cl increased in polar regions during winter as a result of reac- and Br). These gases participate in three principal reaction tions on the surfaces of polar stratospheric clouds (PSCs) (see cycles that destroy ozone. Q8 and Q10). Cycles 2 and 3 (see Figure Q9-2) become the Cycle 1. Ozone destruction Cycle 1 is illustrated in Figure dominant reaction mechanisms for polar ozone loss because of Q9-1. -
Glossary Chem2007.Pdf
An English‐Chinese and Chinese‐English Glossary of Terms Commonly Used in the Teaching of Chemistry in Secondary Schools 中學化學科常用英漢及漢英辭彙 Prepared by the Curriculum Development Council 2007 香港課程發展議會編訂 二零零七年 English-Chinese Glossaries of Terms Commonly Used in the Teaching of Chemistry in Secondary Schools 2007 ID English Chinese 1 (-)-2,3-dihydroxybutanedioic acid (-)-2,3-二羥基丁二酸 2 (—)-tartaric acid (—)-酒石酸 3 (+)-2,3-dihydroxybutanedioic acid (+)-2,3-二羥基丁二酸 4(+)-tartaric acid (+)-酒石酸 5 (2,4-dichlorophenoxy)ethanoic acid (2,4-二氯苯氧基)乙酸 6 (bromomethyl)benzene (溴甲基)苯 7 (chloromethyl)benzene (氯甲基)苯 8 (dichloromethyl)benzene (二氯甲基)苯 9 (trichloromethyl)benzene (三氯甲基)苯 10 cis--but-2-enal 順-丁-2-烯醛 11 cis-but-2-ene 順-丁-2-烯 12 cis-but-2-enoic acid 順-丁-2-烯酸 13 cis-butenedioate 順-丁烯二酸鹽;順-丁烯二酸<某>酯 14 cis-butenedioic acid 順-丁烯二酸 15 cis-butenedioic anhydride 順-丁烯二<酸>酐 16 cis-diamminedichloroplatinum(II) 順-二氨二氯合鉑(II),順-二氯.二氨合鉑(II) 17 cis-methylbutenedioic acid 順-甲基丁烯二酸 18 cis-octadec-9-enoic acid 順-十八碳-9-烯酸 19 d-glucose 右旋葡萄糖 20 d-tartaric acid 右旋酒石酸 21 l-tartaric acid 左旋酒石酸 22 l-glucose 左旋葡萄糖 23 m- (meta-) 間 24 m-cresol 間甲酚 25 m-hydroxybenzoic acid 間羥基苯<甲>酸 26 m-nitrotoluene 間硝基甲苯 27 m-toluic acid 間甲苯<甲>酸 28 m-xylene 間二甲苯 29 meso-2,3-dihydroxybutanedioic acid 內消旋-2,3-二羥基丁二酸 30 meso-tartaric acid 內消旋酒石酸 31 meso-tartrate 內消旋酒石酸鹽 32 N,N-dimethylaniline N,N-二甲基苯胺 33 N,N-dimethylbenzenamine N,N-二甲基苯胺 34 N,N-dimethylethanamide N,N-二甲基乙酰胺 35 N,N-dimethylphenylamine N,N-二甲基苯胺 36 N,N-diethylethanamine N,N-二乙基乙胺 37 N-(bromophenyl)ethanamide N-(溴苯基)乙酰胺 38 N-(nitrophenyl)ethanamide -

SROC Annex V
Annex V Major Chemical Formulae and Nomenclature This annex presents the formulae and nomenclature for halogen-containing species and other species that are referred to in this report (Annex V.1). The nomenclature for refrigerants and refrigerant blends is given in Annex V.2. V.1 Substances by Groupings V.1.1 Halogen-Containing Species V.1.1.1 Inorganic Halogen-Containing Species Atomic chlorine Cl Atomic bromine Br Molecular chlorine Cl2 Molecular bromine Br2 Chlorine monoxide ClO Bromine monoxide BrO Chlorine radicals ClOx Bromine radicals BrOx Chloroperoxy radical ClOO Bromine nitrate BrONO2, BrNO3 Dichlorine peroxide (ClO dimer) (ClO)2, Cl2O2 Potassium bromide KBr Hydrogen chloride (Hydrochloric acid) HCl Inorganic chlorine Cly Antimony pentachloride SbCl5 Atomic fluorine F Molecular fluorine F2 Atomic iodine I Hydrogen fluoride (Hydrofluoric acid) HF Molecular iodine I2 Sulphur hexafluoride SF6 Nitrogen trifluoride NF3 IPCC Boek (dik).indb 467 15-08-2005 10:57:13 468 IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System V.1.1.2 Halocarbons For each halocarbon the following information is given in columns: • Chemical compound [Number of isomers]1 (or common name) • Chemical formula • CAS number2 • Chemical name (or alternative name) V.1.1.2.1 Chlorofluorocarbons (CFCs) CFC-11 CCl3F 75-69-4 Trichlorofluoromethane CFC-12 CCl2F2 75-71-8 Dichlorodifluoromethane CFC-13 CClF3 75-72-9 Chlorotrifluoromethane CFC-113 [2] C2Cl3F3 Trichlorotrifluoroethane CCl FCClF 76-13-1 CFC-113 2 2 1,1,2-Trichloro-1,2,2-trifluoroethane -

HIGH HAZARD GAS Review Date: 09/23/2019
University of Pittsburgh EH&S Guideline Number: 04-021 Safety Manual Subject: Effective Date: 04/19/2017 Page 1 of 9 HIGH HAZARD GAS Review Date: 09/23/2019 STORAGE AND USE OF HIGH HAZARD GAS 1. Definition of High Hazard (HH) Gases For these guidelines, any gas meeting one or more of the following definitions based on International Fire Code (IFC) and National Fire Protection Association (NFPA) standards: 1.1. Flammable gas – a material that is a gas at 68ºF (20ºC) or less at an absolute pressure of 14.7 psi (101.325 kPa) when in a mixture of 13% or less by volume with air, or that has a flammable range at an absolute pressure of 14.7 psi (101.325 kPa) with air of at least 12%, regardless of the lower limit 1.2. Pyrophoric gas – a gas with an autoignition temperature in air at or below 130ºF (54.4ºC) 1.3. Health Hazard 3 (HH3) gas – material that, under emergency conditions and according to the standards, can cause serious or permanent injury 1.4. Health Hazard 4 (HH4) gas – material that, under emergency conditions and according to the standards, can be lethal The storage and usage of a gas or gases meeting any of the above definitions must follow all applicable IFC and NFPA guidelines and the requirements outlined in this document. Consult EH&S for specific guidance on gas mixtures containing corrosive, flammable or poisonous gas components (ex. 1% carbon monoxide/nitrogen, 5% hydrogen sulfide/helium). 2. Notification Requirements Prior to Obtaining High Hazard Gases 2.1. -

Ozone Depletion, Greenhouse Gases, and Climate Change
DOCUMENT RESUME ED 324 229 SE 051 620 TITLE Ozone Depletion, Greenhouse Gaaes, and Climate Change. Proceedings of a Joint Symposium by theBoard on Atmospheric Sciences and Climate andthe Committee on Global Change, National ResearchCouncil (Washington, D.C., March 23, 1988). INSTITUTION National Academy of Sciences - National Research Council, Washington, D.C. SPONS AGENCY National Science Foundation, Washington, D.C. REPORT NO ISBN-0-309-03945-2 PUB DATE 90 NOTE 137p. AVAILABLE FROMNational Academy of Scences, National AcademyPress, 2101 Constitution Avenue, NW, Washington, DC 20418 ($20.00). PUB TYPE Collected Works Conference Proceedings (021) EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIPTORS Air Pollution; *Climate; *Conservation(Environment); Depleted Resources; Earth Science; Ecology; *Environmental Education; *Environmental Influences; Global Approach; *Natural Resources; Science Education; Thermal Environment; World Affairs; World Problems IDENTIFIERS *Global Climate Change ABSTRACT The motivation for the organization of thissymposium was the accumulation of evidence from manysources, both short- and longterm,_that the global climate is in a state of change. Data which defy integrated explanation including temperature, ozone, methane, precipitation and other climate-related trendshave presented troubling problems for atmospheric sciencesince the 1980's. Ten papers from this symposium are presentedhere: (1) "Global Change and the Changing Atmosphere"(William C. Clark); (2) "Stratospheric Ozone Depletion: Global Processes"(Daniel L. Albritton); (3) "Stratospheric Czone Depletion: AntarcticProcesses" (Robert T. Watson); (4) "The Role of Halocarbons in Stratospheric Ozone Depletion" (F. Sherwood Rowland);(5) "Heterogenous Chemical Processes in Ozone Depletion" (Mario J. Molina);(6) "Free Radicals in the Earth's Atmosphere: Measurement andInterpretation" (James G. Anderson); (7) "Theoretical Projections of StratosphericChange Due to Increasing Greenhouse Gases and Changing OzoneConcentrations" (Jerry D. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Ozone Depletion, Chlorine Activation and Water Vapor Observed in Spitsbergen
Ozone depletion, chlorine activation and water vapor observed in Spitsbergen Dissertation zur Erlangung des Grades Dr. rer. nat. der Universitat¨ Bremen vorgelegt von Ingo Wohltmann April 2002 Berichte aus dem Institut fur¨ Umweltphysik – Band 0 herausgegeben von: Dr. Georg Heygster Universitat¨ Bremen, FB 1, Institut fur¨ Umweltphysik, Postfach 33 04 40, D-28334 Bremen URL http://www.iup.physik.uni-bremen.de E-Mail [email protected] Prufer:¨ Prof. Dr. Klaus Kunzi,¨ Prof. Dr. John Burrows c 2001 Ingo Wohltmann Contents 1 Physics and Chemistry of the Atmosphere 13 1.1 Composition of the Atmosphere . 13 1.2 Physics of the Atmosphere . 15 1.3 Dynamics of the Stratosphere . 18 1.4 Ozone Chemistry . 21 1.5 Ozone Distribution . 29 1.6 Chlorine Monoxide and Water Vapor . 31 1.7 Literature . 31 2 Instrument 33 2.1 Overview . 33 2.2 Radiative Transfer . 34 2.3 Absorption Coefficients . 36 2.4 Instrument Description . 40 2.4.1 Quasi Optics . 40 2.4.2 Antenna and Mixer . 42 2.4.3 Intermediate Frequency Chain . 44 2.4.4 Acousto Optical Spectrometer . 45 2.4.5 Bremen Radiometer . 45 2.5 Measurement Principle . 45 2.5.1 Totalpower Method . 46 2.5.2 Reference Beam Method . 47 3 Retrieval 49 3.1 Overview . 49 3.2 Optimal Estimation . 49 3.2.1 Resolution . 51 3.2.2 Errors . 53 3.2.3 Nonlinearities . 54 3.3 Retrieval . 55 3 3.3.1 General Features . 55 3.3.2 Forward Model Features . 59 3.3.3 Moist Weather Conditions . 72 3.4 Water Vapor, Spitsbergen . -

Consolidated List of Chemicals Subject to the Emergency Planning
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response July 2011 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction ....................................................................................................................................... i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ............................................... 1 Appendix A: Alphabetical Listing of Consolidated List ................................................................. A-1 Appendix B: Radionuclides Listed Under CERCLA...................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes ............................................. C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency -
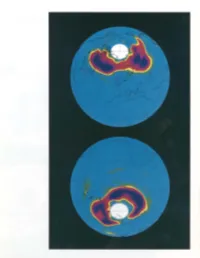
The Chlorine Threat to Stratospheric Ozone
The Chlorine Threat to Stratospheric Ozone Whether we! re in for still more unpleasant sur prises is a very by Joe W. Waters difficult question . to answer. Ozone has a split personality. Down near there would be no ozone in the atmosphere, and Earth's surface, it's a bad guy-an element of reciprocally, that ozone now shields life from pollution and smog-which those of us who live solar ultraviolet radiation. in the los Angeles area are all too familiar with. A molecule of ozone consists ~f three atoms In the stratosphere, however, where most of the of oxygen bound together, represented as 03' ozone resides, it's a good guy-in fact, its Its abundance in the stratosphere is relatively Chlorine monoxide presence there is essential for life on Earth. Here slight---onlyabout 10 molecules per million (CIO), the form of I'm going to discuss only the good-guy ozone and total molecules at maximum. But that small chlorine mainly implicated in the how it's threatened by chlorine, currently a very abundance is a very effective absorber of solar destruction of strato· active area of research. Tremendous progress is ultraviolet radiation. Ultraviolet radiation, a very spheric ozone, as being made in understanding this phenomenon, short wavelength of light, is very damaging to measured on Febru· ary 14, 1993, in the but in the history of this research scientists have life, a principal manifestation in humans being northern hemisphere, been unpleasantly surprised by the processes that skin cancer. It has been estimated that a 5- and on August 14, can deplete ozone.