Piccolo® Comprehensive Metabolic Reagent Disc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WELLNESS SCREENING TEST EXPLANATION SHEET We Encourage You to Share All Lab Results with Your Primary Care Provider
WELLNESS SCREENING TEST EXPLANATION SHEET We encourage you to share all lab results with your primary care provider. EXPLANATION OF BLOOD TESTS HEMOGLOBIN A1C: A Hemoglobin A1c level is HEMATOLOGY WELLNESS: expressed as a percent and is used to evaluate the Blood counts are often used as a broad screening test average amount of glucose in the blood over the last to determine an individual’s general health status. 2 to 3 months. This test may be used to screen for and White blood cells are the cells that are part of the diagnose diabetes or risk of developing diabetes. body’s defense system against infections and cancer and also play a role in allergies and inflammation. ALT: This test is used to determine if a patient has Red blood cells are the cells that transport oxygen liver damage. In healthy individuals, ALT levels in the throughout the body. Hemoglobin measures the blood are low and they rise in various disease states. total amount of the oxygen-carrying protein in the blood, which generally reflects the number of red CALCIUM: A calcium test is ordered to screen for, blood cells in the blood. Hematocrit measures the diagnose and monitor a range of conditions percentage of a person’s total blood volume that relating to the bones, heart, nerves, kidneys and consists of red blood cells. Platelets are cell fragments teeth. The test may also be ordered if a person has that are vital for normal blood clotting. symptoms of parathyroid disorder, malabsorption or an overactive thyroid. FREE T4: Free T4 is used to help evaluate thyroid function, generally after determining the TSH is CREATININE: The creatinine blood test is used to abnormal. -

Diagnosis of Insulinoma in a Maine Coon
JOR0010.1177/2055116919894782Journal of Feline Medicine and Surgery Open ReportsGifford et al 894782research-article2020 Case Report Journal of Feline Medicine and Surgery Open Reports Diagnosis of insulinoma in 1 –10 © The Author(s) 2020 Article reuse guidelines: a Maine Coon cat sagepub.com/journals-permissions DOI:https://doi.org/10.1177/2055116919894782 10.1177/2055116919894782 journals.sagepub.com/home/jfmsopenreports This paper was handled and processed by the American Editorial Office (AAFP) 1 2 3 Carol H Gifford , Anita P Morris , Kurt J Kenney and for publication in JFMS Open Reports J Scot Estep4 Abstract Case summary A 9-year-old male neutered Maine Coon cat presented with a 6-month history of polyphagia and one recent episode of tremors and weakness. Blood work revealed profound hypoglycemia and results of a paired insulin glucose test were consistent with an insulinoma. Abdominal ultrasound revealed a solitary pancreatic mass, and results of a fine-needle aspirate (FNA) gave further support for the location of the neuroendocrine tumor. After unsuccessful medical management of the hypoglycemia, the mass was surgically removed. Immunohistochemistry confirmed that it was an insulinoma. At the time of writing, the patient had been in clinical remission for 9 months. Relevance and novel information Feline insulinomas are rare and there is very little information on their behavior, clinical course and histologic characteristics. This is the first reported case of an insulinoma in a Maine Coon cat and the first to describe results of an ultrasound-guided FNA of the mass. In addition, the progression of disease, histopathology and immunohistochemistry results add to the currently minimal database for feline insulinomas. -

Levels of Serum Uric Acid in Patients with Impaired Glucose Tolerance
JOURNAL OF CRITICAL REVIEWS ISSN- 2394-5125 VOL 08, ISSUE 02, 2021 LEVELS OF SERUM URIC ACID IN PATIENTS WITH IMPAIRED GLUCOSE TOLERANCE 1,2Shahzad Ali Jiskani*, 1Lubna Naz, 3Ali Akbar Pirzado, 4Dolat Singh, 5Asadullah Hanif, 6Razia Bano 1Department of Pathology, Chandka Medical College, SMBBMU, Larkano 2Department of Pathology, Indus Medical College Hospital, Tando Muhammad Khan 3Department of Community Medicine and Public Health, Chadka Medical College, SMBBMU, Larkana 4Department of Medicine, Indus Medical College Hospital, Tando Muhammad Khan 5Health Department, Govt. of Sindh 6Department of Medicine, Liaquat University of Medical & Health Sciences Jamshoro *Corresponding Author: Shahzad Ali Jiskani Department of Pathology, Chandka Medical College, SMBBMU, Larkano ABSTRACT Objective: The main objective of this study is to see levels of serum uric acid in patients having impaired glucose tolerance as compared to normal glucose tolerant individuals. Patients and Methods: This was prospective study, conducted at Department of Medicine and Department of Pathology, Indus Medical College Hospital, Tando Muhammad Khan for the period of 8 months (March 2019 to October 2019). A total of 140 patients were included in this study. An oral glucose test was performed with load of 75g glucose in patients having impaired glucose in fasting. Both normal glucose tolerance and impaired glucose tolerance individuals were evaluated for their serum uric acid level. Serum glucose levels and serum uric acid levels were performed on COBAS C-111 Chemistry Analyzer ensuring procedures of quality control. Data was entered and analyzed using SPSS 21.0. Results: In patients having impaired glucose tolerance, the frequency of hyperuricemia was 62.35%. Analysis of both groups (patients with normal glucose tolerance and glucose intolerance), association was strong with p-value of <0.001. -

C-Reactive Protein and Gamma-Glutamyltransferase
Hindawi Publishing Corporation Experimental Diabetes Research Volume 2010, Article ID 761715, 8 pages doi:10.1155/2010/761715 Clinical Study C-Reactive Protein and Gamma-Glutamyltransferase Concentrations in Relation to the Prevalence of Type 2 Diabetes Diagnosed by Glucose or HbA1c Criteria in Chinese Adults in Qingdao, China J. Ren,1 Z. C. Pang,2 W. G. Gao,3, 4, 5 H. R. Nan,6 S. J. Wang,2 L. Zhang,3, 4, 5 and Q. Qiao3, 4 1 Department of Epidemiology and Health Statistics, Shandong University, Jinan 250012, China 2 Department of Non-Communicable Disease Prevention, Qingdao Municipal Centre for Disease Control and Prevention, no. 175, Shandong Road, Qingdao 266071, China 3 Department of Public Health, University of Helsinki, 00014 Helsinki, Finland 4 Diabetes Unit, Department of Chronic Disease Prevention, National Institute for Health and Welfare, 00014 Helsinki, Finland 5 Qingdao Endocrinology and Diabetes Hospital, 266071 Qingdao, China 6 Hong Kong Institute of Diabetes and Obesity, Hong Kong, China CorrespondenceshouldbeaddressedtoZ.C.Pang,[email protected] Received 13 August 2010; Revised 12 October 2010; Accepted 12 October 2010 Academic Editor: Giuseppe Paolisso Copyright © 2010 J. Ren et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aims. To investigate the association of C-reactive protein (CRP) and gamma glutamyltransferase (GGT) concentrations with newly diagnosed diabetes defined by either glucose or HbA1c criteria in Chinese adults. Methods. A population-based cross-sectional study was conducted in 2006. Data from 1167 men and 1607 women aged 35–74 years were analyzed. -
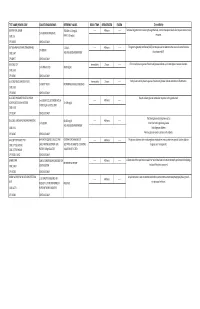
G's- Lab Test Menu
TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN Clinical Utility GENTAMICIN, SERUM TROUGH: 0-2.0 mg/dL ------ 48 hours ------ Tests level of gentamicin circulating through the body. Certain therapeutic levels elicit require anti-microbial 2 ml SERUM. REFRIGERATE. CODE: GE PEAK: 5-10 mg/dL response. CPT: 81070 SCHEDULE: DAILY GGT (GAMMA GLUTAMYL TRANSFERASE) 5-31 IU/L ------ 48 hours ------ The gamma-glutamyl transferase (GGT) test may be used to determine the cause of elevated alkaline 1ml SERUM CODE: GGT AGE AND GENDER DEPENDENT phosphatase (ALP). CPT:82977 SCHEDULE: DAILY GLUCOSE, CSF Immediately 2 hours ------ CFS normally has no glucose. Presence of glucose indicates spinal meningitis or seizure disorders. 1 ml SPINAL FLUID 40-80 mg/mL CODE: GLFL CPT: 82947 SCHEDULE: DAILY GLUCOSE, MISCELLANEOUS FLUID Immediately 2 hours ------ Body fluids normally have no glucose. Presence of glucose indicates infection or inflammation. 1 ml BODY FLUID NO NORMAL RANGE ESTABLISHED CODE: GLFL CPT: 82945 SCHEDULE: DAILY GLUCOSE, PREGNANT RELATED 1 HOUR Results indicate glucose metabolism response to the glucola load. 1 ml SERUM COLLECTED ONE HOUR ------ 48 hours ------ LOADING (O'SULLIVAN SCREEN) 70-139 mg/dL AFTER 50 gm GLUCOSE LOAD CODE: GESC CPT: 82947 SCHEDULE: DAILY The blood glucose test may be used to: GLUCOSE, SERUM (FASTING AND RANDOM) 60-115 mg/dL ------ 48 hours ------ 1 ml SERUM Screen for both high blood glucose AGE AND GENDER DEPENDENT CODE: GLU Help diagnose diabetes CPT: 82947 SCHEDULE: DAILY Monitor glucose levels in persons with diabetes GLUCOSE TOLERANCE TEST 8HR FAST REQUIRED. COLLECT AND CRITERIA FOR DIAGNOSIS OF ------ 48 hours ------ The glucose tolerance test monitors glucose metabolism over a certain time period. -

(Insulin Dependent) Diabetes Mellitus Is Related to Poor Residual Cell Function
410 Archives ofDisease in Childhood 1996;75:410-415 Ketoacidosis at the diagnosis of type 1 (insulin Arch Dis Child: first published as 10.1136/adc.75.5.410 on 1 November 1996. Downloaded from dependent) diabetes mellitus is related to poor residual cell function Jorma Komulainen, Raisa Lounamaa, Mikael Knip, Eero A Kaprio, Hans K Akerblom, and the Childhood Diabetes in Finland Study Group Abstract observed to be associated with age at diagno- The determinants of the degree of meta- sis,' degree of metabolic decompensation,9 bolic decompensation at the diagnosis of mild clinical symptoms at diagnosis,2910 and type 1 (insulin dependent) diabetes melli- strict initial blood glucose control.213 Diabetic tus (IDDM) and the possible role of ketoacidosis is a serious consequence of insuf- diabetic ketoacidosis in the preservation ficient insulin secretion.'4 In addition to possi- and recovery of residual P cell function ble acute complications, it may also influence were examined in 745 Finnish children the later outcome of diabetes. and adolescents. Children younger than 2 To study the effect of age, sex, and years or older than 10 years of age were socioeconomic factors on the clinical condition found to be more susceptible to diabetic ofthe patient at the diagnosis of IDDM, and to ketoacidosis than children between 2 and find out whether metabolic decompensation at 10 years of age (<2 years: 53.3%; 2-10 diagnosis is related to subsequent endogenous years: 16.9%; >10 years: 33.3%). Children insulin secretion and impaired metabolic con- from families with poor parental trol, 745 Finnish children and adolescents, educational level had ketoacidosis more aged 0.8-14.9 years, were evaluated at the time often than those from families with high of diagnosis of IDDM and then observed for parental educational level (24.4% v two years. -

Clinical Performance of Flash Glucose Monitoring System in Patients With
www.nature.com/scientificreports OPEN Clinical Performance of Flash Glucose Monitoring System in Patients with Liver Cirrhosis and Diabetes Mellitus Dalila Costa1,2 ✉ , Joana Lourenço1, Ana Margarida Monteiro3, Beatriz Castro1, Patricia Oliveira3, Maria Carmo Tinoco3, Vera Fernandes3, Olinda Marques3, Raquel Gonçalves2 & Carla Rolanda1,2 Flash glucose monitoring system (FGMS) is an improved subset of continuous glucose monitoring with a recognized efectiveness on glycemic control, though validation in patients with Liver Cirrhosis (LC) is lacking. To evaluate the accuracy of FGMS in patients with Type 2 Diabetes Mellitus (DM) and LC, a prospective, case-control study was performed in 61 ambulatory patients with LC and DM (LC group, n = 31) or DM (Control group, n = 30). During 14 days, patients performed 4 assessments per day of self-monitoring of blood glucose (SMBG, reference value) followed by FGMS scanning. There were 2567 paired SMBG and FGMS values used in the accuracy analysis, with an overall mean absolute relative diference (MARD) of 12.68% in the LC group and 10.55% in the control group (p < 0,001). In patients with LC, the percentage of readings within Consensus Consensus Error Grid analysis Zone A and A + B were 80.36% and 99,26%, respectively. Sensor clinical accuracy was not afected by factors such as body mass index, age, gender, Child-Pugh score or edematoascitic decompensation. This is the frst study to approach FGMS clinical accuracy in LC, revealing a potential usability of this system to monitor glycemic control in this population. Type 2 Diabetes Mellitus (DM) has been considered a Public Health challenge due to its signifcant impact on mortality and morbidity worldwide1. -

Hyperchloremia – Why and How
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):347–353 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Brief review Hyperchloremia – Why and how Glenn T. Nagami Nephrology Section, Department of Medicine, VA Greater Los Angeles Healthcare System and David Geffen School of Medicine at UCLA, United States a r t i c l e i n f o a b s t r a c t Article history: Hyperchloremia is a common electrolyte disorder that is associated with a diverse group of Received 5 April 2016 clinical conditions. The kidney plays an important role in the regulation of chloride concen- Accepted 11 April 2016 tration through a variety of transporters that are present along the nephron. Nevertheless, Available online 3 June 2016 hyperchloremia can occur when water losses exceed sodium and chloride losses, when the capacity to handle excessive chloride is overwhelmed, or when the serum bicarbonate is low Keywords: with a concomitant rise in chloride as occurs with a normal anion gap metabolic acidosis Hyperchloremia or respiratory alkalosis. The varied nature of the underlying causes of the hyperchloremia Electrolyte disorder will, to a large extent, determine how to treat this electrolyte disturbance. Serum bicarbonate Published by Elsevier Espana,˜ S.L.U. on behalf of Sociedad Espanola˜ de Nefrologıa.´ This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/). -

Journal of Gynecology & Reproductive Medicine
Research Article Journal of Gynecology & Reproductive Medicine The Association Between Hyperuricemia in first Trimester and the Development of Gestational Diabetes Mounir Mohammed Fawzy, Mohamed El-Mandooh Mohamed, Alaa Sayed Hassanin and Mina Nashaat Ghally1* *Corresponding author Resident of Obstetrics and Gynecology, Al-Qussia central Hospital. Mina Nashaat Ghally, Resident of Obstetrics andGynecology, Al-Qussia central Hospital, Egypt. Submitted: 30 June 2017; Accepted: 03 July 2017; Published: 05 July 2017 Abstract Objective: This study aimed to assess the accuracy of the elevation of serum uric acid in the first trimester as a predictive test for development of gestational diabetes mellitus. Patientsand methods: It was a prospective observational study included 200 pregnant patients who were regularly attending the out-patient clinic for routine antenatal care to find if the elevated first trimester uric acid is associated with development of GDM or not. Results: The risk of developing GDM was higher if first-trimester uric acid was < 3.1 mg/dI. Women who developed GDM were significantly older when they compared to women who did not develop GDM [Normal with Mean + SD 24.53 + 4.40 years, Abnormal with Mean + SD 32.78 + 8.18 years, p-value 0.016]. It was found that, the mean BMI was significantly higher in women who developed GDM when compared to women who did not develop GDM [Normal 115 cases of total sample and no one developed GD with Mean + SD, 95.57 + 12.32, Overweight 59 cases, 56 cases (29.3%) were normal and 3 cases (33.3%) had GD with Mean + SD 106.29 + 26.62, Obese 26 cases, 20 cases (10.5%) were normal and 6 cases (66.7%) had GD with Mean + SD 124.27 + 39.78, p-value 0.000]. -

Bicarbonate Therapy in Severe Metabolic Acidosis
CLINICAL COMMENTARY www.jasn.org Bicarbonate Therapy in Severe Metabolic Acidosis Sandra Sabatini and Neil A. Kurtzman Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas ABSTRACT monly termed “potential” bicarbonate. The utility of bicarbonate administration to patients with severe metabolic acidosis Giving bicarbonate to a patient with a remains controversial. Chronic bicarbonate replacement is obviously indicated for true bicarbonate deficit is not controver- patients who continue to lose bicarbonate in the ambulatory setting, particularly sial. Controversy arises when the de- patients with renal tubular acidosis syndromes or diarrhea. In patients with acute crease in bicarbonate concentration is lactic acidosis and ketoacidosis, lactate and ketone bodies can be converted back the result of its conversion to another to bicarbonate if the clinical situation improves. For these patients, therapy must base, which, given time, can be converted be individualized. In general, bicarbonate should be given at an arterial blood pH back to bicarbonate. If one knew that the of Յ7.0. The amount given should be what is calculated to bring the pH up to 7.2. timely and efficient conversion of aceto- The urge to give bicarbonate to a patient with severe acidemia is apt to be all but acetate and -hydroxybutyrate or lactate irresistible. Intervention should be restrained, however, unless the clinical situation back to bicarbonate would occur with- clearly suggests benefit. Here we discuss the pros and cons of bicarbonate therapy out morbidity or mortality, then there for patients with severe metabolic acidosis. would be no reason even to contemplate giving bicarbonate. J Am Soc Nephrol 20: 692–695, 2009. -
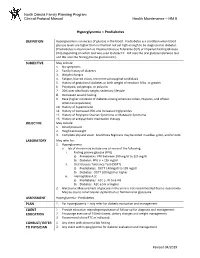
Hyperglycemia – Prediabetes
North Dakota Family Planning Program Clinical Protocol Manual Health Maintenance – HM 8 Hyperglycemia – Prediabetes DEFINITION Hyperglycemia is an excess of glucose in the blood. Prediabetes is a condition when blood glucose levels are higher than normal but not yet high enough to be diagnosed as diabetes. (Prediabetes is also known as Impaired Glucose Tolerance (IGT) or Impaired Fasting Glucose (IFG) depending on which test was used to detect it. IGT uses the oral glucose tolerance test and IFG uses the fasting plasma glucose test.) SUBJECTIVE May include: 1. No symptoms 2. Family history of diabetes 3. Weight changes 4. Fatigue, blurred vision, recurrent vulvovaginal candidiasis 5. History of gestational diabetes or birth weight of newborn 9 lbs. or greater 6. Polydipsia, polyphagia, or polyuria 7. 20% over ideal body weight; sedentary lifestyle 8. Decreased wound healing 9. Race (higher incidence of diabetes among American Indian, Hispanic, and African American population) 10. History of hypertension 11. History of decreased HDL and increased triglycerides 12. History of Polycystic Ovarian Syndrome or Metabolic Syndrome 13. History of antipsychotic medication therapy OBJECTIVE May include: 1. Blood pressure 2. Height and weight 3. Complete physical exam. Acanthosis Nigricans may be noted in axillae, groin, and/or neck LABORATORY May refer for: 1. Hyperglycemia a. lab of choice may include one or more of the following: i. Fasting plasma glucose (FPG) a) Prediabetes: FPG between 100 mg/dl to 125 mg/dl b) Diabetes: FPG is > 126 mg/dl ii. Oral Glucose Tolerance Test (OGTT) a) Prediabetes: OGTT 140mg/dl to 199 mg/dl b) Diabetes: OGTT 200mg/dl or higher iii. -

Association Between Sodium Bicarbonate Consumption and Human Health: a Systematic Review
Available online at www.ijmrhs.com International Journal of Medical Research & ISSN No: 2319-5886 Health Sciences, 2016, 5, 8:22-29 Association between sodium bicarbonate consumption and human health: A systematic review Yadolah Fakhri 1, Nazak Amanidaz 2, Yahya Zandsalimi 3, Maryam Dadar 4, Ali Moradi 5, Bigard Moradi 6, Leila Rasouli Amirhajeloo 7, Hassan Keramati 8, Athena Rafieepour 9,* 1Food and Cosmetic Health Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran 2Environmental Health Research Center, Golestan University of Medical Sciences, Golestan, Iran 3Environmental Health Research Center, Kurdistan University of Medical Sciences, Sanandaj, Iran 4Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Karaj, Iran 5Asadabad Health and Treatment Network, Hamadan University of Medical Sciences, Hamadan, Iran 6Department of Health Public, Kermanshah University of Medical Sciences, Kermanshah, Iran 7Department of Environmental Health Engineering, School of Public Health, Qom University of Medical Sciences, Qom, Iran 8Department of Environmental Health Engineering, School of Public Health, Semnan University of Medical Sciences, Semnan, Iran 9Studends Research Office, Shahid Beheshti University of Medical sciences, Tehran, Iran *Correspondence Email: [email protected] _____________________________________________________________________________________________ ABSTRACT Sodium bicarbonate or baking soda is a chemical compound dissolved in water which is widely used as an additive in foods and mineral water and as a medicine. In Iran, due to the introduction of harmful effects of this compound, using it in baking is prohibited. Therefore, we tried to search and evaluate all health effects of using this compound with a systematic review. In this study, all available evidences on the beneficial and harmful effects of sodium bicarbonate were searched.