Bicarbonate Therapy in Severe Metabolic Acidosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Insulin Dependent) Diabetes Mellitus Is Related to Poor Residual Cell Function
410 Archives ofDisease in Childhood 1996;75:410-415 Ketoacidosis at the diagnosis of type 1 (insulin Arch Dis Child: first published as 10.1136/adc.75.5.410 on 1 November 1996. Downloaded from dependent) diabetes mellitus is related to poor residual cell function Jorma Komulainen, Raisa Lounamaa, Mikael Knip, Eero A Kaprio, Hans K Akerblom, and the Childhood Diabetes in Finland Study Group Abstract observed to be associated with age at diagno- The determinants of the degree of meta- sis,' degree of metabolic decompensation,9 bolic decompensation at the diagnosis of mild clinical symptoms at diagnosis,2910 and type 1 (insulin dependent) diabetes melli- strict initial blood glucose control.213 Diabetic tus (IDDM) and the possible role of ketoacidosis is a serious consequence of insuf- diabetic ketoacidosis in the preservation ficient insulin secretion.'4 In addition to possi- and recovery of residual P cell function ble acute complications, it may also influence were examined in 745 Finnish children the later outcome of diabetes. and adolescents. Children younger than 2 To study the effect of age, sex, and years or older than 10 years of age were socioeconomic factors on the clinical condition found to be more susceptible to diabetic ofthe patient at the diagnosis of IDDM, and to ketoacidosis than children between 2 and find out whether metabolic decompensation at 10 years of age (<2 years: 53.3%; 2-10 diagnosis is related to subsequent endogenous years: 16.9%; >10 years: 33.3%). Children insulin secretion and impaired metabolic con- from families with poor parental trol, 745 Finnish children and adolescents, educational level had ketoacidosis more aged 0.8-14.9 years, were evaluated at the time often than those from families with high of diagnosis of IDDM and then observed for parental educational level (24.4% v two years. -

Hyperchloremia – Why and How
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):347–353 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Brief review Hyperchloremia – Why and how Glenn T. Nagami Nephrology Section, Department of Medicine, VA Greater Los Angeles Healthcare System and David Geffen School of Medicine at UCLA, United States a r t i c l e i n f o a b s t r a c t Article history: Hyperchloremia is a common electrolyte disorder that is associated with a diverse group of Received 5 April 2016 clinical conditions. The kidney plays an important role in the regulation of chloride concen- Accepted 11 April 2016 tration through a variety of transporters that are present along the nephron. Nevertheless, Available online 3 June 2016 hyperchloremia can occur when water losses exceed sodium and chloride losses, when the capacity to handle excessive chloride is overwhelmed, or when the serum bicarbonate is low Keywords: with a concomitant rise in chloride as occurs with a normal anion gap metabolic acidosis Hyperchloremia or respiratory alkalosis. The varied nature of the underlying causes of the hyperchloremia Electrolyte disorder will, to a large extent, determine how to treat this electrolyte disturbance. Serum bicarbonate Published by Elsevier Espana,˜ S.L.U. on behalf of Sociedad Espanola˜ de Nefrologıa.´ This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/). -

Association Between Sodium Bicarbonate Consumption and Human Health: a Systematic Review
Available online at www.ijmrhs.com International Journal of Medical Research & ISSN No: 2319-5886 Health Sciences, 2016, 5, 8:22-29 Association between sodium bicarbonate consumption and human health: A systematic review Yadolah Fakhri 1, Nazak Amanidaz 2, Yahya Zandsalimi 3, Maryam Dadar 4, Ali Moradi 5, Bigard Moradi 6, Leila Rasouli Amirhajeloo 7, Hassan Keramati 8, Athena Rafieepour 9,* 1Food and Cosmetic Health Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran 2Environmental Health Research Center, Golestan University of Medical Sciences, Golestan, Iran 3Environmental Health Research Center, Kurdistan University of Medical Sciences, Sanandaj, Iran 4Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Karaj, Iran 5Asadabad Health and Treatment Network, Hamadan University of Medical Sciences, Hamadan, Iran 6Department of Health Public, Kermanshah University of Medical Sciences, Kermanshah, Iran 7Department of Environmental Health Engineering, School of Public Health, Qom University of Medical Sciences, Qom, Iran 8Department of Environmental Health Engineering, School of Public Health, Semnan University of Medical Sciences, Semnan, Iran 9Studends Research Office, Shahid Beheshti University of Medical sciences, Tehran, Iran *Correspondence Email: [email protected] _____________________________________________________________________________________________ ABSTRACT Sodium bicarbonate or baking soda is a chemical compound dissolved in water which is widely used as an additive in foods and mineral water and as a medicine. In Iran, due to the introduction of harmful effects of this compound, using it in baking is prohibited. Therefore, we tried to search and evaluate all health effects of using this compound with a systematic review. In this study, all available evidences on the beneficial and harmful effects of sodium bicarbonate were searched. -

The Effect of Potassium on Intracellular Bicarbonate in Slices of Kidney Cortex
THE EFFECT OF POTASSIUM ON INTRACELLULAR BICARBONATE IN SLICES OF KIDNEY CORTEX Helen M. Anderson, … , Gilbert H. Mudge, Theodora J. Lannon J Clin Invest. 1955;34(11):1691-1697. https://doi.org/10.1172/JCI103222. Research Article Find the latest version: https://jci.me/103222/pdf THE EFFECT OF POTASSIUM ON INTRACELLULAR BICARBON- ATE IN SLICES OF KIDNEY CORTEX1 By HELEN M. ANDERSON AND GILBERT H. MUDGE 2 WITH THE TECHNICAL ASSISTANCE OF THEODORA J. LANNON (From the Department of Medicine, College of Physicians and Surgeons, Columbia University, and the Presbyterian Hospital, New York City, N. Y.) (Submitted for publication June 21, 1955; accepted July 20, 1955) An increase in serum bicarbonate concentration concentrations in the kidney cortex can be varied has been observed in association with potassium over a wide range by the use of in vitro techniques, depletion in a variety of clinical and experimental studies of tissue slices were undertaken in order to conditions. Although the development of the al- define the relationships of intracellular potassium kalosis may be extra-renal in origin, its perpetua- and bicarbonate. tion must depend upon an alteration in renal func- tion which can be characterized as either an in- METHODS crease in bicarbonate reabsorption or an increase The preparation of tissues has been described previously in hydrogen ion excretion. Many instances of so- in detail (7) and is summarized here. Fresh slices were made from the renal cortex of rabbits killed by carotid called "paradoxical" aciduria have been described exsanguination and were depleted of potassium by leach- (cf. 1, 2). ing for one hour in 0.15 N NaCl at room temperature. -
Understanding Your Lab Results: Comprehensive Metabolic Screening Panel
Understanding Your Lab Results: Comprehensive Metabolic Screening Panel CHOLESTEROL and TRIGLYCERIDES are fats necessary for normal cell function. However, elevated levels of these fats have been associated with an increased risk of developing coronary disease, arteriosclerosis, and heart attack. A patient’s dietary status, medications, presence of illness, lifestyle, and family history may represent factors influencing cholesterol levels. The signifi- cance of cholesterol levels should be determined within the context of each individual patient. If your cholesterol level is 200 mg/dl or greater, please consult your physician. Triglyceride levels greater than 150 mg/dl, in a true fasting specimen, are considered elevated. In this case, please consult your physician. HDL (High-Density Lipoprotein) CHOLESTEROL, commonly known as the “GOOD” cholesterol, picks up cholesterol and transports it for removal from the body. The higher the HDL value, the lower the risk of developing coronary disease, arterioscle- rosis, and heart attack. LDL (Low-Density Lipoprotein) CHOLESTEROL, commonly known as the “BAD” cholesterol, picks up cholesterol and transports it to the cells of the body for storage. Desirable LDL levels are less than 130 mg/dl. The higher the LDL value, the greater the risk of developing coronary disease, arteriosclerosis, and heart attack. CHOLESTEROL/HDL RATIO is a calculation used to predict an increased or decreased risk of cardiovascular disease relative to a normal. The higher the ratio, the greater the risk of developing coronary disease, arteriosclerosis, and heart attack. GLUCOSE, commonly called a blood sugar, is the transport form of carbohydrates in the body as they move to storage or to utiliza- tion. -
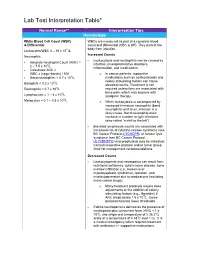
Lab Test Interpretation Table*
Lab Test Interpretation Table* Normal Range** Interpretation Tips Hematology White Blood Cell Count (WBC) WBCs are measured as part of a complete blood & Differential count and differential (CBC & diff). They protect the body from infection. Leukocytes/WBC 4 – 10 x 109 /L Increased Counts Neutrophils - Leukocytosis and neutrophilia can be caused by Absolute Neutrophil Count (ANC) = - infection, myeloproliferative disorders, 2 – 7.5 x 109/L inflammation, and medications. - Calculated ANC = WBC x (segs+bands) / 100 o In cancer patients, supportive - Band neutrophils: < 0.7 x 109/L medications such as corticosteroids and 9 colony stimulating factors can cause Basophils < 0.2 x 10 /L elevated counts. Treatment is not Eosinophils < 0.7 x 109/L required unless they are associated with 9 bone pain, which may improve with Lymphocytes = 1 – 4 x 10 /L analgesic therapy. 9 Monocytes = 0.1 – 0.8 x 10 /L o When leukocytosis is accompanied by increased immature neutrophils (band neutrophils) and fever, infection is a likely cause. Band neutrophils often increase in number to fight infections (also called “a shift to the left”). - Elevated lymphocyte counts are associated with increased risk of cytokine-release syndrome (see BC Cancer Protocol LYCHOPR) or tumour lysis syndrome (see BC Cancer Protocol ULYVENETO) and prophylaxis may be indicated. Consult respective protocol and/or tumor group chair for management recommendations. Decreased Counts - Leukocytopenia and neutropenia can result from nutritional deficiency, autoimmune disease, bone marrow infiltration (i.e., leukemia or myelodysplastic syndrome), radiation, and myelosuppression due to medications (including many cancer drugs). o Many treatment protocols require dose adjustments or the addition of colony stimulating factors (e.g., filgrastim) if ANC drops below 1.5 x 109/L. -

Dementia Evaluation: Lab Testing
Dementia Evaluation: Lab Testing This material is provided by UCSF Weill Institute for Neurosciences as an educational resource for patients. Models for illustrative purposes only. Dementia Evaluation: Lab Testing How do lab tests help with my diagnosis? Interpretation High TSH indicates an underactive thyroid (“hypothyroidism”) Certain blood tests can help evaluate for treatable conditions that Low TSH indicates an overactive thyroid (“hyperthyroidism”) may be contributing to changes in thinking or memory. Vitamin B12 What lab tests will my provider order? Vitamin B12 deficiency is common, particularly with increasing age, and can cause a variety of symptoms including cognitive Your provider will review any existing lab tests you have had done and/or psychiatric symptoms. It can also cause anemia (low and may order some new tests. There are certain blood tests that blood cell count), imbalance and numbness/tingling in the toes. are recommended for anyone with changes in thinking or memory If your blood level of vitamin B12 is borderline, your doctor may and others that are performed on an individual basis, according to order an additional lab test called methylmalonic acid (MMA), your own personal medical history and risk factors. Below are some which is an indirect measure of the amount of functional B12 of the most common blood tests ordered as part of a diagnostic you have in your system. A high MMA level indicates evaluation for someone with changes in thinking or memory. insufficient B12. CBC (complete blood count) Interpretation This lab test measures the numbers of white blood cells (WBCs, Low B12 level indicates vitamin B12 deficiency which fight infection), red blood cells (RBCs, which carry oxygen), High MMA indicates vitamin B12 deficiency and platelets (plts, which help blood clot) that are present in your blood. -

Blood Test Results: CMP Explained
Blood Test Results: CMP Explained Comprehensive Metabolic Panel (CMP) Definition: Measures kidney and liver function, electrolyte levels Substance What It Is Reference Ranges * What a Low Number May Mean What a High Number May Mean USA UK/EU Australia/Canada Glucose (fasting or non-fasting) Sugar in the blood 70-99 mg/dL (fasting) Hypoglycemia, liver disease, adrenal insufficiency, excess insulin Hyperglycemia, certain types of diabetes, prediabetes, 70-125 mg/dL (non-fasting) pancreatitis, hyperthyroidism Sodium (Na) An electrolyte which keeps your body in balance 136-144 mEq/L Use of diuretics, diarrhea, adrenal insufficiency Kidney dysfunction, dehydration, Cushing's syndrome Potassium (K) An electrolyte and mineral 3.7-5.2 mEq/L Use of diuretics or corticosteroids (such as prednisone or Acute or chronic kidney failure, Addison's disease, diabetes, cortisone dehydration Chloride (Cl) An electrolyte 96-106 mmol/L Emphysema, chronic lung disease Dehydration, Cushing's syndrome, kidney disease Carbon dioxide (bicarbonate) (CO2) Gaseous waste product from metabolism 20-29 mmol/L Kidney disease, certain toxic exposures, severe infection Lung diseases, including COPD BUN (blood urea nitrogen) A waste product formed in the liver and carried to the kidneys, filtered out 7-20 mg/dL Malnutrition Liver or kidney disease, heart failure of blood, and excreted through urine Creatinine A chemical waste produced by muscle metabolism 0.8-1.4 mg/dL Low muscle mass, malnutrition Chronic or temporary decrease in kidney function BUN/creatinine ratio 10:1 -

Piccolo® Comprehensive Metabolic Reagent Disc
Piccolo® Comprehensive Metabolic Panel For In Vitro Diagnostic Use and For Professional Use Only Customer and Technical Service: 1-800-822-2947 Applicable to US customers only Customers outside the US: +49 6155 780 210 CLIA Waived: Use lithium heparin whole blood, only Moderate Complexity: Use lithium heparin whole blood, lithium heparin plasma , or serum Abaxis, Inc. ABAXIS Europe GmbH 3240 Whipple Rd. Bunsenstr. 9-11 Union City, CA 94587 64347 Griesheim USA Germany 1. Intended Use The Piccolo® Comprehensive Metabolic Panel, used with the Piccolo® blood chemistry analyzer or the Piccolo Xpress® chemistry analyzer, is intended to be used for the in vitro quantitative determination of alanine aminotransferase (ALT), albumin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), calcium, chloride, creatinine, glucose, potassium, sodium, total bilirubin, total carbon dioxide, total protein, and blood urea nitrogen (BUN) in heparinized whole blood, heparinized plasma, or serum in a clinical laboratory setting or point-of-care location. For US Customers Only The tests on this panel are waived under CLIA ’88 regulations. If a laboratory modifies the test system instructions, then the tests are considered high complexity and subject to all CLIA requirements. For CLIA waived labs, only lithium heparin whole blood may be tested. For use in moderate complexity labs, lithium heparinized whole blood, lithium heparinized plasma, or serum may be used. A CLIA Certificate of Waiver is needed to perform CLIA waived testing. A Certificate of Waiver can be obtained from the Centers for Medicare & Medicaid Services (CMS). Please contact the Commission on Laboratory Accreditation (COLA) at 1- 800-981-9883 for assistance in obtaining one. -

Potassium Bicarbonate Crops 1 2 Identification of Petitioned Substance 3 4 Chemical Names: 16 Trade Names: 5 17 Purple K, Kaligreen 6 CHKO3 (MW
Potassium Bicarbonate Crops 1 2 Identification of Petitioned Substance 3 4 Chemical Names: 16 Trade Names: 5 17 Purple K, Kaligreen 6 CHKO3 (MW. 100.12), Potassium hydrogen 18 7 carbonate, potassium acid carbonate, CAS Numbers: 8 potassium ion bicarbonate 298-14-6 9 10 Other Name: Other Codes: 11 19 SID 162102857, MDL Number 12 Carbonic Acid, potassium salt, Carbonic 20 MFCD00011402, EINECS 206-059-0, 13 acid, monopotassium salt, Carbonic acid, 21 PubChem Substance ID 24854226, Beilstein 14 monopotassium salt bicarbonate 22 Registry Number 4535309 15 23 Summary of Current Use 24 25 As required by the Organic Foods Production Act, the National Organic Standards Board has the 26 responsibility to review each substance on the National List within five years of its adoption or 27 previous review. A previous technical report for potassium bicarbonate was completed in July, 28 2002 and is available on the NOP website (NOP, 2002). For the 2017 sunset review, the NOSB 29 requested an updated limited scope technical evaluation report for potassium bicarbonate 30 covering alternatives to its use. To support their decision-making the document has been limited 31 to the following sections: 32 Identification of the Petitioned Substance 33 Summary of Current Use 34 Evaluation Question #11 35 Evaluation Question #12 36 The current listing for potassium bicarbonate is scheduled to sunset on 6/27/2017 (USDA NOP, 37 2013). 38 The National Organic Standards Board (NOSB) voted unanimously to allow the use of potassium 39 bicarbonate for plant disease control at its October 25-28, 1999 meeting (USDA AMS, 1999; USDA 40 NOP 1999). -

Structure and Metabolism of Natural and Synthetic Bilirubins
Original Article &&&&&&&&&&&&&& Structure and Metabolism of Natural and Synthetic Bilirubins David A. Lightner studies.2,3 Yet, although the ridge-tile shape is far more stable than Antony F. McDonagh the conventional linear representation, or even a porphyrin-like shape, the bilirubin ridge tile is not rigid. It may flex and bend, and flutter lepidopterously — and even invert to the mirror image ridge tile — all the while maintaining most, if not all, of its 3 The secondary structure of bilirubin, with a ridge-tile shape and six intramolecular hydrogen bonds. And, just as knowledge of intramolecular hydrogen bonds, is more stable than any other conformation stereochemistry, especially the handedness of molecules, has recently and perhaps the most important determinant of its solubility and properties become the sine qua non for current studies in the efficacy and in solution and its hepatic metabolism/excretion. Uncoupling the metabolism of pharmacophores, so it, too, is having an impact on intramolecular hydrogen bonding, increasing the acidity of the propionic our understanding of bile pigment metabolism. acid, or even increasing its length can decrease the pigment’s It is apparently the tight embrace of propionic acid and hydrophobicity and permit its excretion intact across the liver into bile; less dipyrrinone, by hydrogen bonding, that dictates the hydrophobicity of intuitively obvious, so can subtle modifications at C(10), the pivotal carbon bilirubin-IX (or simply bilirubin) and its inability to be secreted in the ridge tile. (Taken from a talk given by D.A.L. at the International intact, across the liver from blood to bile. -

L7 -Management of Diabetic Ketoacidosis and Hypoglycemia Characters of DKA
King Saud University College of Medicine 2nd Year, Endocrine Block L7 -Management of diabetic ketoacidosis and hypoglycemia Characters of DKA Diabetic Diagnostic Ketoacidosis criteria in DKA Lines of treatment Mind map Characters and causes of hypoglycemia Hypoglycemia Precautions Treatment of hypoglycemia slide doctor’s note important explanation Diabetic Ketoacidosis Metabolic Changes : Its a serious acute emergency • Carbohydrates situation with a risk of death. • Increase Glycogenolysis • Increase Gluconeogenesis • Protein Develops as a result of insulin deficiency • Increase proteolysis thus providing amino acid for gluconeogenesis. • Fats A characteristic feature of type I diabetes (could occur with • Increase Lipolysis& ketogenesis type II (especially during • Fat > free fatty acids > acetyl-CoA > stress)) ketone bodies > Ketonemia > Ketonuria & Acidosis • acetoacetic acid, β-hydroxybutyric acid and acetone (↑ ketogenesis ). slide doctor’s note important explanation Diabetic ketoacidosis Hyperglycemia induced Symptoms: Character of DKA: glucosuria, osmotic diuresis & severe fluid loss. -Thirst, Polyuria, Polydipsia -Hyperglycemia. Fluid loss induces dehydration & (increased drinking). -Glucosuria . electrolyte imbalance. Metabolic acidosis induces -Nausea, Vomiting, -Osmotic diuresis. hyperventilation. Abdominal pain -Polyuria. -Tachycardia, -Thirst. Diagnostic Criteria: -Polydipsia (increased drinking). – Blood glucose level > 250 -Kussmaul–Kien respiration (rapid & deep), Ketotic -Dehydration. mg/dl breath (fruity, with