BIOLOGICAL FIELD STATION Cooperstown, New York
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NON-TIDAL BENTHIC MONITORING DATABASE: Version 3.5
NON-TIDAL BENTHIC MONITORING DATABASE: Version 3.5 DATABASE DESIGN DOCUMENTATION AND DATA DICTIONARY 1 June 2013 Prepared for: United States Environmental Protection Agency Chesapeake Bay Program 410 Severn Avenue Annapolis, Maryland 21403 Prepared By: Interstate Commission on the Potomac River Basin 51 Monroe Street, PE-08 Rockville, Maryland 20850 Prepared for United States Environmental Protection Agency Chesapeake Bay Program 410 Severn Avenue Annapolis, MD 21403 By Jacqueline Johnson Interstate Commission on the Potomac River Basin To receive additional copies of the report please call or write: The Interstate Commission on the Potomac River Basin 51 Monroe Street, PE-08 Rockville, Maryland 20850 301-984-1908 Funds to support the document The Non-Tidal Benthic Monitoring Database: Version 3.0; Database Design Documentation And Data Dictionary was supported by the US Environmental Protection Agency Grant CB- CBxxxxxxxxxx-x Disclaimer The opinion expressed are those of the authors and should not be construed as representing the U.S. Government, the US Environmental Protection Agency, the several states or the signatories or Commissioners to the Interstate Commission on the Potomac River Basin: Maryland, Pennsylvania, Virginia, West Virginia or the District of Columbia. ii The Non-Tidal Benthic Monitoring Database: Version 3.5 TABLE OF CONTENTS BACKGROUND ................................................................................................................................................. 3 INTRODUCTION .............................................................................................................................................. -

Otsego County Soil & Water Conservation Di
_________________________________________________________________________________ Otsego County Soil & Water Conservation District 967 CO HWY 33 – RIVER ROAD – COOPERSTOWN, NEW YORK 13326-9222 – PHONE (607) 547-8337 ext. 4 OTSEGO COUNTY SWCD BOARD MEETING MINUTES Thursday, June 20, 2019 Members Present: Staff Present: Les Rathbun, Chair, Grange Rep. Jordan Clements, District Mgr. Meg Kennedy, Vice Chair, Cty. Rep. Sherry Mosher, District Secretary Roseboom Sr, Farm Bureau Michelle Farwell, Cty. Rep. Absent: Ed Lentz, Member @ Large Doris Moennich, Land owner Guest: None I. -Les called the meeting to order @ 10:00 am. II. –Approval of May Minutes, motion to approve made by Michelle, seconded by Meg, seconded by Larry. III. -Approval of May treasurer report, motion to approve made by Michelle, seconded by Larry. - Approval of paid bills, motion to approve made by Meg, seconded by Larry. IV. – District Reports: Sherry -Sherry stated that she opened a new checking account for Part C funds only, allowing separate designated line item names with their balances. -The new credit cards arrived for Mark & Jessica. -Sherry asked the board for approval to attend a 2 day QuickBooks training in Albany for the updated QuickBooks pro 2019, a motion was made to approve by Larry and 2nd by Michelle, motion carried. -District Reports: Jordan: -Jordan stated that he would like a resolution to apply for the NRCS CIG grant (Conservation Innovation Grant). The federal grant would be getting money for implementing cover crops with a self-propelled sprayer, renting -

Town of Otsego Comprehensive Plan Appendices
Town of Otsego Comprehensive Plan Appendices Draft (V6) March 2007 Town of Otsego Comprehensive Plan – Draft March 2007 Table of Contents Appendix A Consultants Recommendations to Implement Plan A1 Appendix B 2006 Update: Public Input B1 Appendix C 2006 Update: Profile and Inventory of Town Resources C1 Appendix D Zoning Build-out Analysis D1 Appendix E Strengths, Weaknesses, Opportunities and Threats Analysis E1 Appendix F 1987 Master Plan F1 Appendix G Ancillary Maps G1 See separate document for Comprehensive Plan: Section 1 Introduction Section 2 Summary of Current Conditions and Issues Section 3 Vision Statement Section 4 Goals Section 5 Strategies to Implement Goals Section 6 Mapped Resources Appendix A Consultants Recommendations to Implement Plan APPENDIX A-1 Town of Otsego Comprehensive Plan – Draft March 2007 Appendix A. Consultants Recommendations to Implement Plan This section includes strategies, actions, policy changes, programs and planning recommendations presented by the consultants (included in the plan as reference materials) that could be undertaken by the Town of Otsego to meet the goals as established in this Plan. They are organized by type of action. Recommended Strategies Regulatory and Project Review Initiatives 1. Utilize the Final GEIS on the Capacities of the Cooperstown Region in decision making in the Town of Otsego. This document analyzes and identifies potential environmental impacts to geology, aquifers, wellhead protection areas, surface water, Otsego Lake and Watershed, ambient light conditions, historic resources, visual resources, wildlife, agriculture, on-site wastewater treatment, transportation, emergency services, demographics, economic conditions, affordable housing, and tourism. This document will offer the Planning Board and other Town agencies, background information, analysis, and mitigation to be used to minimize environmental impacts of future development. -

A Characterization of the Riparian Corridor of the Oaks Creek Blueway Trail with Emphasis on Otsego Land Trust Properties
A characterization of the riparian corridor of the Oaks Creek Blueway Trail with emphasis on Otsego Land Trust properties Nicole Pedisich1 and Donna Vogler2 INTRODUCTION The Otsego Land Trust Blueway is a series of Land Trust owned and protected parcels that provide fishing, hiking, paddling, bird watching, and educational opportunities from Canadarago Lake to the Susquehanna River including Brookwood Point on Otsego Lake. (Otsego Land Trust 2014). The trail consists of Fetterly Forest, Deowongo Island, Oaks Creek Preserve, Crave, Parslow Road, Greenough Road, and Compton Bridge. For this project, an assessment of the riparian vegetation communities of Oaks Creek was conducted along a section of the Blueway Trail starting in Schuyler Lake and ending in Cattown. More in-depth characterizations of plant communities were done at Oaks Creek Preserve, the Crave property, and Parslow Road Conservation Area. Oaks Creek is a stream located in Otsego County, NY. It flows from Canadarago Lake southeast into the Susquehanna River, a distance of approximately 13.8 miles. (Hingula 2004). A majority of the stretch of stream assessed is state-regulated freshwater wetlands (Figure 1, NYSDEC). Oaks Creek Preserve is a 28-acre parcel located along its namesake between Schuyler Lake and Oaksville. Downstream are Crave, a parcel recently acquired by the Otsego Land Trust and Parslow Road Conservation Area, an 86-acre parcel located on the northern edge of Oaksville running a half-mile along Oaks Creek (Figure 2). 3 1 BFS Intern, summer 2015. Current affiliation: SUNY College at Oneonta. Funding for this project was provided by the Otsego Land Trust. 2 Professor. -

Susquehanna Riyer Drainage Basin
'M, General Hydrographic Water-Supply and Irrigation Paper No. 109 Series -j Investigations, 13 .N, Water Power, 9 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY CHARLES D. WALCOTT, DIRECTOR HYDROGRAPHY OF THE SUSQUEHANNA RIYER DRAINAGE BASIN BY JOHN C. HOYT AND ROBERT H. ANDERSON WASHINGTON GOVERNMENT PRINTING OFFICE 1 9 0 5 CONTENTS. Page. Letter of transmittaL_.__.______.____.__..__.___._______.._.__..__..__... 7 Introduction......---..-.-..-.--.-.-----............_-........--._.----.- 9 Acknowledgments -..___.______.._.___.________________.____.___--_----.. 9 Description of drainage area......--..--..--.....-_....-....-....-....--.- 10 General features- -----_.____._.__..__._.___._..__-____.__-__---------- 10 Susquehanna River below West Branch ___...______-_--__.------_.--. 19 Susquehanna River above West Branch .............................. 21 West Branch ....................................................... 23 Navigation .--..........._-..........-....................-...---..-....- 24 Measurements of flow..................-.....-..-.---......-.-..---...... 25 Susquehanna River at Binghamton, N. Y_-..---...-.-...----.....-..- 25 Ghenango River at Binghamton, N. Y................................ 34 Susquehanna River at Wilkesbarre, Pa......_............-...----_--. 43 Susquehanna River at Danville, Pa..........._..................._... 56 West Branch at Williamsport, Pa .._.................--...--....- _ - - 67 West Branch at Allenwood, Pa.....-........-...-.._.---.---.-..-.-.. 84 Juniata River at Newport, Pa...-----......--....-...-....--..-..---.- -

Otsego County Water Quality Coordinating Committee Annual Report 2010 & 2011
Otsego County Water Quality Coordinating Committee Annual Report 2010 & 2011 Prepared by: Otsego County Water Quality Coordinating Committee 967 County Highway 33 Cooperstown, NY 13326 6/18/2012 Table of Contents INTRODUCTION OF WATER QUALITY COORDINATING COMMITTEE 2 COORDINATING COMMITTEE MEMBERS 2 COMMITTEE MISSION, PURPOSE AND PRIMARY FUNCTIONS 3 2010 ACCOMPLISHMENTS 5 2011 ACCOMPLISHMENTS 7 ATTACHMENTS: #1 Water Quality Coordinating Committee By Laws #2 2011 Otsego County Nonpoint Source Water Quality Strategy #3 Susquehanna Headwaters Nutrient Report #4 Otsego County Soil & Water Conservation District 2011 Report #5 Otsego County Conservation Association 2011 Report #6 Goodyear Lake Association 2011 Report #7 Otsego Lake Watershed Supervisory Committee 2011 Report #8 SUNY Oneonta Biological Field Station 2011 Staff Activity Report #9 Village of Richfield 2011 Water Quality Report 1 INTRODUCTION OTSEGO COUNTY WATER QUALITY COORDINATING COMMITTEE Non-point pollution (NPS) by definition is any form of pollution not being discharged from a distinguished point or source. Sources of NPS, being so diffuse and variable, will often require multi-agency involvement to remediate the existing water pollution problems. Understanding that the responsibility and interest in water quality issues are represented by a wide range of parties, a committee was formed and is known as the Otsego County Water Quality Coordinating Committee (the Committee) and functions as a subcommittee of the Otsego County Soil and Water Conservation District. The Committee is responsible for the preparation of the Otsego County Non-point Source Water Quality Strategy. The Strategy attempts to ensure that the individual efforts of local, county, state, and federal agencies regarding water quality programs and educational outreach events are coordinated for maximum effectiveness. -

Section 4: County Profile
SECTION 4: COUNTY PROFILE SECTION 4: COUNTY PROFILE Broome County profile information is presented in the plan and analyzed to develop an understanding of a study area, including the economic, structural, and population assets at risk and the particular concerns that may be present related to hazards analyzed later in this plan (e.g., low lying areas prone to flooding or a high percentage of vulnerable persons in an area). This profile provides general information for Broome County (physical setting, population and demographics, general building stock, and land use and population trends) and critical facilities located within the County. GENERAL INFORMATION Broome County is a rural community located within the south-central part or “Southern Tier” of New York State. The Southern Tier is a geographical term that refers to the counties of New York State that lie west of the Catskill Mountains, along the northern border of Pennsylvania. Broome County lies directly west of Delaware County, 137 miles southwest of Albany and approximately 177 miles northwest of New York City. Broome County occupies approximately 715 square miles and has a population of approximately 199,031 (U.S. Census, 2011). Broome County is one of the 62 counties in New York State and is comprised of one city, sixteen towns, seven villages and many hamlets. The City of Binghamton is the County seat and is located at the confluence of the Susquehanna and Chenango Rivers. The City of Binghamton is part of the “Triple Cities,” which also includes the Villages of Endicott and Johnson City. With two Interstates and a major state route intersecting in the City of Binghamton, the area is the crossroads of the Southern Tier. -

Brook Trout Outcome Management Strategy
Brook Trout Outcome Management Strategy Introduction Brook Trout symbolize healthy waters because they rely on clean, cold stream habitat and are sensitive to rising stream temperatures, thereby serving as an aquatic version of a “canary in a coal mine”. Brook Trout are also highly prized by recreational anglers and have been designated as the state fish in many eastern states. They are an essential part of the headwater stream ecosystem, an important part of the upper watershed’s natural heritage and a valuable recreational resource. Land trusts in West Virginia, New York and Virginia have found that the possibility of restoring Brook Trout to local streams can act as a motivator for private landowners to take conservation actions, whether it is installing a fence that will exclude livestock from a waterway or putting their land under a conservation easement. The decline of Brook Trout serves as a warning about the health of local waterways and the lands draining to them. More than a century of declining Brook Trout populations has led to lost economic revenue and recreational fishing opportunities in the Bay’s headwaters. Chesapeake Bay Management Strategy: Brook Trout March 16, 2015 - DRAFT I. Goal, Outcome and Baseline This management strategy identifies approaches for achieving the following goal and outcome: Vital Habitats Goal: Restore, enhance and protect a network of land and water habitats to support fish and wildlife, and to afford other public benefits, including water quality, recreational uses and scenic value across the watershed. Brook Trout Outcome: Restore and sustain naturally reproducing Brook Trout populations in Chesapeake Bay headwater streams, with an eight percent increase in occupied habitat by 2025. -

Northeast PA Rivers and Watersheds
contents JUNE 2012 a n e C x e l 20 A 7 92 7 2012 Green Leaders 88 Show Pop He’s Tops! Happenings honors those who Find creative ways to show Dad protect our region’s waterways. you care in the Father’s Day Gift Guide. 16 Natural Attractions Discover natural wonders in 98 Men’s Health at All Ages your own backyard. Meet healthy men of every age, and learn expert tips for staying 20 River Guide well. Find highlights of Northeast PA rivers and watersheds. 122 Better Together Join in on the fun of reunions 36 Golf Guide hosted in this region. See where to tee off this summer! 134 June is Jumping! 41 Explore More & Win! Things to do, where to go, Enter to win tickets to The National everything you need to know! Baseball Hall of Fame and Museum! 60 History Revisited Learn how history in Hawley is shaping the town’s future through major development projects. 76 All Things Automotive Mark your calendar for car-centric events; peek inside a classic car, and prepare for summer travel. June 2012 HappeningsMagazinePA.com 3 MAILBAG Dear Happenings, FROM THE ASSOCIATE EDITOR Your magazine is a monthly read for my family and Publisher Paula Rochon Mackarey me. And it is a wonderful resource for our entertain- ment and source of local retailers. Managing Editor Barbara Toolan –Kathy Sallavanti (via email) Art Director Lisa M. Ragnacci DearIt’s the sixth Readers, year Happenings is honoring still think spending time on the rocky shore… Northeast PA’s Green Leaders! This year, we where you can’t download an app, skype or Associate Art Director Peter Salerno Dear Happenings, put the focus on water preservation.We’ve even get a text message… is good for one’s I absolutely love Administrative Assistant Katherine Kempa chosen the 2012 Green Leaders (on pages 7- soul. -
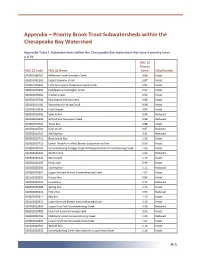
Appendix – Priority Brook Trout Subwatersheds Within the Chesapeake Bay Watershed
Appendix – Priority Brook Trout Subwatersheds within the Chesapeake Bay Watershed Appendix Table I. Subwatersheds within the Chesapeake Bay watershed that have a priority score ≥ 0.79. HUC 12 Priority HUC 12 Code HUC 12 Name Score Classification 020501060202 Millstone Creek-Schrader Creek 0.86 Intact 020501061302 Upper Bowman Creek 0.87 Intact 020501070401 Little Nescopeck Creek-Nescopeck Creek 0.83 Intact 020501070501 Headwaters Huntington Creek 0.97 Intact 020501070502 Kitchen Creek 0.92 Intact 020501070701 East Branch Fishing Creek 0.86 Intact 020501070702 West Branch Fishing Creek 0.98 Intact 020502010504 Cold Stream 0.89 Intact 020502010505 Sixmile Run 0.94 Reduced 020502010602 Gifford Run-Mosquito Creek 0.88 Reduced 020502010702 Trout Run 0.88 Intact 020502010704 Deer Creek 0.87 Reduced 020502010710 Sterling Run 0.91 Reduced 020502010711 Birch Island Run 1.24 Intact 020502010712 Lower Three Runs-West Branch Susquehanna River 0.99 Intact 020502020102 Sinnemahoning Portage Creek-Driftwood Branch Sinnemahoning Creek 1.03 Intact 020502020203 North Creek 1.06 Reduced 020502020204 West Creek 1.19 Intact 020502020205 Hunts Run 0.99 Intact 020502020206 Sterling Run 1.15 Reduced 020502020301 Upper Bennett Branch Sinnemahoning Creek 1.07 Intact 020502020302 Kersey Run 0.84 Intact 020502020303 Laurel Run 0.93 Reduced 020502020306 Spring Run 1.13 Intact 020502020310 Hicks Run 0.94 Reduced 020502020311 Mix Run 1.19 Intact 020502020312 Lower Bennett Branch Sinnemahoning Creek 1.13 Intact 020502020403 Upper First Fork Sinnemahoning Creek 0.96 -

BIOLOGICAL FIELD STATION Cooperstown, New York
BIOLOGICAL FIELD STATION Cooperstown, New York 50th ANNUAL REPORT 2017 Photo credit: Holly Waterfield STATE UNIVERSITY OF NEW YORK COLLEGE AT ONEONTA OCCASIONAL PAPERS PUBLISHED BY THE BIOLOGICAL FIELD STATION No. 1. The diet and feeding habits of the terrestrial stage of the common newt, Notophthalmus viridescens (Raf.). M.C. MacNamara, April 1976 No. 2. The relationship of age, growth and food habits to the relative success of the whitefish (Coregonus clupeaformis) and the cisco (C. artedi) in Otsego Lake, New York. A.J. Newell, April 1976. No. 3. A basic limnology of Otsego Lake (Summary of research 1968-75). W. N. Harman and L. P. Sohacki, June 1976. No. 4. An ecology of the Unionidae of Otsego Lake with special references to the immature stages. G. P. Weir, November 1977. No. 5. A history and description of the Biological Field Station (1966-1977). W. N. Harman, November 1977. No. 6. The distribution and ecology of the aquatic molluscan fauna of the Black River drainage basin in northern New York. D. E Buckley, April 1977. No. 7. The fishes of Otsego Lake. R. C. MacWatters, May 1980. No. 8. The ecology of the aquatic macrophytes of Rat Cove, Otsego Lake, N.Y. F. A Vertucci, W. N. Harman and J. H. Peverly, December 1981. No. 9. Pictorial keys to the aquatic mollusks of the upper Susquehanna. W. N. Harman, April 1982. No. 10. The dragonflies and damselflies (Odonata: Anisoptera and Zygoptera) of Otsego County, New York with illustrated keys to the genera and species. L.S. House III, September 1982. -

Water Quality Monitoring of Five Major Tributaries in the Otsego Lake Watershed, Summer 2000
17 Water quality monitoring of five major tributaries in the Otsego Lake watershed, summer 2000 Mary M. Miner l INTRODUCTION For the past three decades, research conducted by the Biological Field Station has recognized trends toward eutrophy in Otsego Lake (e.g. Harman et al., 1997). Recent studies have continued to document these trends (Albright, 1998;1999; 2000). These include algal blooms which lead to undesirable tastes, odors and colors, pose health concerns to those who use the lake as a source ofdrinking. Dissolved organics resulting from increased algal blooms react with chlorine used to disinfect drinking water to form carcinogens (Harman et a!., 1997). Low dissolved oxygen concentrations also raise concern as they jeopardize the cold water fisheries ofthe lake. Water flowing into the lake is the key factor for the character ofthe lake; the quality ofinflowing water is dependent upon landforms and land used within the watershed (Albright, 1996). This study continues ongoing monitoring ofthe northern watershed ofOtsego Lake, which contributes two-thirds ofthe water flowing into the lake. Otsego Lake has a U·shaped basin with a north-south orientation. It lies in the glacially over-deepened Susquehanna River valley. The northern watershed consists of five drainage basins: those drained by White Creek, Cripple Creek, Hayden Creek, Shadow Brook and a small tributary on Mount Wellington. Problems exist in this part of the watershed due to cultural development and agriculture activities on nutrient-rich soils (Harman et al., 1997). Nutrients from agricultural non-point sources have been identified as the main cause ofcultural eutrophication in freshwater inland lakes ofthe U.S.