Cerebral Medulloepithelioma with Long Survival —Case Report—
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Central Nervous System Tumors General ~1% of Tumors in Adults, but ~25% of Malignancies in Children (Only 2Nd to Leukemia)
Last updated: 3/4/2021 Prepared by Kurt Schaberg Central Nervous System Tumors General ~1% of tumors in adults, but ~25% of malignancies in children (only 2nd to leukemia). Significant increase in incidence in primary brain tumors in elderly. Metastases to the brain far outnumber primary CNS tumors→ multiple cerebral tumors. One can develop a very good DDX by just location, age, and imaging. Differential Diagnosis by clinical information: Location Pediatric/Young Adult Older Adult Cerebral/ Ganglioglioma, DNET, PXA, Glioblastoma Multiforme (GBM) Supratentorial Ependymoma, AT/RT Infiltrating Astrocytoma (grades II-III), CNS Embryonal Neoplasms Oligodendroglioma, Metastases, Lymphoma, Infection Cerebellar/ PA, Medulloblastoma, Ependymoma, Metastases, Hemangioblastoma, Infratentorial/ Choroid plexus papilloma, AT/RT Choroid plexus papilloma, Subependymoma Fourth ventricle Brainstem PA, DMG Astrocytoma, Glioblastoma, DMG, Metastases Spinal cord Ependymoma, PA, DMG, MPE, Drop Ependymoma, Astrocytoma, DMG, MPE (filum), (intramedullary) metastases Paraganglioma (filum), Spinal cord Meningioma, Schwannoma, Schwannoma, Meningioma, (extramedullary) Metastases, Melanocytoma/melanoma Melanocytoma/melanoma, MPNST Spinal cord Bone tumor, Meningioma, Abscess, Herniated disk, Lymphoma, Abscess, (extradural) Vascular malformation, Metastases, Extra-axial/Dural/ Leukemia/lymphoma, Ewing Sarcoma, Meningioma, SFT, Metastases, Lymphoma, Leptomeningeal Rhabdomyosarcoma, Disseminated medulloblastoma, DLGNT, Sellar/infundibular Pituitary adenoma, Pituitary adenoma, -

What Are Brain and Spinal Cord Tumors in Children? ● Types of Brain and Spinal Cord Tumors in Children
cancer.org | 1.800.227.2345 About Brain and Spinal Cord Tumors in Children Overview and Types If your child has just been diagnosed with brain or spinal cord tumors or you are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Are Brain and Spinal Cord Tumors in Children? ● Types of Brain and Spinal Cord Tumors in Children Research and Statistics See the latest estimates for new cases of brain and spinal cord tumors in children in the US and what research is currently being done. ● Key Statistics for Brain and Spinal Cord Tumors in Children ● What’s New in Research for Childhood Brain and Spinal Cord Tumors? What Are Brain and Spinal Cord Tumors in Children? Brain and spinal cord tumors are masses of abnormal cells in the brain or spinal cord 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 that have grown out of control. Are brain and spinal cord tumors cancer? In most other parts of the body, there's an important difference between benign (non- cancerous) tumors and malignant tumors (cancers1). Benign tumors do not invade nearby tissues or spread to distant areas, and are almost never life threatening in other parts of the body. Malignant tumors (cancers) are so dangerous mainly because they can spread throughout the body. Brain tumors rarely spread to other parts of the body, though many of them are considered malignant because they can spread through the brain and spinal cord tissue. But even so-called benign tumors can press on and destroy normal brain tissue as they grow, which can lead to serious or sometimes even life-threatening damage. -

Risk-Adapted Therapy for Young Children with Embryonal Brain Tumors, High-Grade Glioma, Choroid Plexus Carcinoma Or Ependymoma (Sjyc07)
SJCRH SJYC07 CTG# - NCT00602667 Initial version, dated: 7/25/2007, Resubmitted to CPSRMC 9/24/2007 and 10/6/2007 (IRB Approved: 11/09/2007) Activation Date: 11/27/2007 Amendment 1.0 dated January 23, 2008, submitted to CPSRMC: January 23, 2008, IRB Approval: March 10, 2008 Amendment 2.0 dated April 16, 2008, submitted to CPSRMC: April 16, 2008, (IRB Approval: May 13, 2008) Revision 2.1 dated April 29, 2009 (IRB Approved: April 30, 2009 ) Amendment 3.0 dated June 22, 2009, submitted to CPSRMC: June 22, 2009 (IRB Approved: July 14, 2009) Activated: August 11, 2009 Amendment 4.0 dated March 01, 2010 (IRB Approved: April 20, 2010) Activated: May 3, 2010 Amendment 5.0 dated July 19, 2010 (IRB Approved: Sept 17, 2010) Activated: September 24, 2010 Amendment 6.0 dated August 27, 2012 (IRB approved: September 24, 2012) Activated: October 18, 2012 Amendment 7.0 dated February 22, 2013 (IRB approved: March 13, 2013) Activated: April 4, 2013 Amendment 8.0 dated March 20, 2014. Resubmitted to IRB May 20, 2014 (IRB approved: May 22, 2014) Activated: May 30, 2014 Amendment 9.0 dated August 26, 2014. (IRB approved: October 14, 2014) Activated: November 4, 2014 Un-numbered revision dated March 22, 2018. (IRB approved: March 27, 2018) Un-numbered revision dated October 22, 2018 (IRB approved: 10-24-2018) RISK-ADAPTED THERAPY FOR YOUNG CHILDREN WITH EMBRYONAL BRAIN TUMORS, HIGH-GRADE GLIOMA, CHOROID PLEXUS CARCINOMA OR EPENDYMOMA (SJYC07) Principal Investigator Amar Gajjar, M.D. Division of Neuro-Oncology Department of Oncology Section Coordinators David Ellison, M.D., Ph.D. -

Malignant CNS Solid Tumor Rules
Malignant CNS and Peripheral Nerves Equivalent Terms and Definitions C470-C479, C700, C701, C709, C710-C719, C720-C725, C728, C729, C751-C753 (Excludes lymphoma and leukemia M9590 – M9992 and Kaposi sarcoma M9140) Introduction Note 1: This section includes the following primary sites: Peripheral nerves C470-C479; cerebral meninges C700; spinal meninges C701; meninges NOS C709; brain C710-C719; spinal cord C720; cauda equina C721; olfactory nerve C722; optic nerve C723; acoustic nerve C724; cranial nerve NOS C725; overlapping lesion of brain and central nervous system C728; nervous system NOS C729; pituitary gland C751; craniopharyngeal duct C752; pineal gland C753. Note 2: Non-malignant intracranial and CNS tumors have a separate set of rules. Note 3: 2007 MPH Rules and 2018 Solid Tumor Rules are used based on date of diagnosis. • Tumors diagnosed 01/01/2007 through 12/31/2017: Use 2007 MPH Rules • Tumors diagnosed 01/01/2018 and later: Use 2018 Solid Tumor Rules • The original tumor diagnosed before 1/1/2018 and a subsequent tumor diagnosed 1/1/2018 or later in the same primary site: Use the 2018 Solid Tumor Rules. Note 4: There must be a histologic, cytologic, radiographic, or clinical diagnosis of a malignant neoplasm /3. Note 5: Tumors from a number of primary sites metastasize to the brain. Do not use these rules for tumors described as metastases; report metastatic tumors using the rules for that primary site. Note 6: Pilocytic astrocytoma/juvenile pilocytic astrocytoma is reportable in North America as a malignant neoplasm 9421/3. • See the Non-malignant CNS Rules when the primary site is optic nerve and the diagnosis is either optic glioma or pilocytic astrocytoma. -

New Jersey State Cancer Registry List of Reportable Diseases and Conditions Effective Date March 10, 2011; Revised March 2019
New Jersey State Cancer Registry List of reportable diseases and conditions Effective date March 10, 2011; Revised March 2019 General Rules for Reportability (a) If a diagnosis includes any of the following words, every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report the case to the Department in accordance with the provisions of N.J.A.C. 8:57A. Cancer; Carcinoma; Adenocarcinoma; Carcinoid tumor; Leukemia; Lymphoma; Malignant; and/or Sarcoma (b) Every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report any case having a diagnosis listed at (g) below and which contains any of the following terms in the final diagnosis to the Department in accordance with the provisions of N.J.A.C. 8:57A. Apparent(ly); Appears; Compatible/Compatible with; Consistent with; Favors; Malignant appearing; Most likely; Presumed; Probable; Suspect(ed); Suspicious (for); and/or Typical (of) (c) Basal cell carcinomas and squamous cell carcinomas of the skin are NOT reportable, except when they are diagnosed in the labia, clitoris, vulva, prepuce, penis or scrotum. (d) Carcinoma in situ of the cervix and/or cervical squamous intraepithelial neoplasia III (CIN III) are NOT reportable. (e) Insofar as soft tissue tumors can arise in almost any body site, the primary site of the soft tissue tumor shall also be examined for any questionable neoplasm. NJSCR REPORTABILITY LIST – 2019 1 (f) If any uncertainty regarding the reporting of a particular case exists, the health care facility, physician, dentist, other health care provider or independent clinical laboratory shall contact the Department for guidance at (609) 633‐0500 or view information on the following website http://www.nj.gov/health/ces/njscr.shtml. -
OVERVIEW of POSTER QUICK PITCHES (QP) Short Oral Poster Presentations (Max 3 Min Each) Followed by Discussion Time at the End
(draft release date 20 May 2021) OVERVIEW OF POSTER QUICK PITCHES (QP) Short oral poster presentations (max 3 min each) followed by discussion time at the end. Time: Daily from 12:00-13:00 hrs | Format: Zoom webinars Monday 31 May: QP 1-4 Tuesday 1 June: QP 5-9 Wednesday 2 June: QP 10-14 Thursday 3 June: QP 15-18 Monday 31 May Quick Pitch 1. Gliomas Chairs: Pieter Wesseling, Signe Regner Michaelsen QP 1.1 P088. Unexpected presentation of an IDH-mutant glioblastoma at Cheryl Coulter autopsy QP 1.2 P089. PAX2 immunoexpression in adult glioblastoma Montserrat Arumí-Uría QP 1.3 P090. The evaluation of palisading necrosis, vascular endothelial Neşe Yeldir proliferation morphology using digital pathology systems and their relations with prognosis QP 1.4 P107. Prognostic role of the proliferation marker Ki-67 in glioblastomas Rikke Hedegaard Dahlrot taking tumor microenvironment, MGMT promoter methylation and post-surgical treatment into account QP 1.5 P109. Stem cell marker ALDH1A3 is not involved in mediating Julian Allgeier radiochemoresistance while RSL-3 induces ferroptotic cell death in C6 glioma model QP 1.6 P110. Differential expression of genes modulating RNA-methylation in Simon Deacon low- and high-grade glioma QP 1.7 P112. The role of aldehyde dehydrogenase 1 (ALDH1) in the treatment of Alicia Gantzkow glioma cells with cold atmospheric plasma (CAP) QP 1.8 P113. Prognostic impact of CDKN2A/B genes deletion and MGMT Maria Fernanda Ruiz promoter gene deletion on diffuse astrocytic tumors QP 1.9 P114. Utility of the new molecular platforms in the study of complex Miguel Idoate pediatric brain tumors QP 1.10 P116. -

WHO Classification of Tumors of the Central Nervous System
Appendix A: WHO Classification of Tumors of the Central Nervous System WHO Classification 1979 • Ganglioneuroblastoma • Anaplastic [malignant] gangliocytoma and Zülch KJ (1979) Histological typing of tumours ganglioglioma of the central nervous system. 1st ed. World • Neuroblastoma Health Organization, Geneva • Poorly differentiated and embryonal tumours • Glioblastoma Tumours of Neuroepithelial tissue –– Variants: • Astrocytic tumours –– Glioblastoma with sarcomatous compo- • Astrocytoma nent [mixed glioblastoma and sarcoma] –– fibrillary –– Giant cell glioblastoma –– protoplasmic • Medulloblastoma –– gemistocytic –– Variants: • Pilocytic astrocytoma –– desmoplastic medulloblastoma • Subependymal giant cell astrocytoma [ven- –– medullomyoblastoma tricular tumour of tuberous sclerosis] • Medulloepithelioma • Astroblastoma • Primitive polar spongioblastoma • Anaplastic [malignant] astrocytoma • Gliomatosis cerebri • Oligodendroglial tumours • Oligodendroglioma Tumours of nerve sheath cells • Mixed-oligo-astrocytoma • Neurilemmoma [Schwannoma, neurinoma] • Anaplastic [malignant] oligodendroglioma • Anaplastic [malignant] neurilemmoma [schwan- • Ependymal and choroid plexus tumours noma, neurinoma] • Ependymoma • Neurofibroma –– Myxopapillary • Anaplastic [malignant]neurofibroma [neurofi- –– Papillary brosarcoma, neurogenic sarcoma] –– Subependymoma • Anaplastic [malignant] ependymoma Tumours of meningeal and related tissues • Choroid plexus papilloma • Meningioma • Anaplastic [malignant] choroid plexus papil- –– meningotheliomatous [endotheliomatous, -
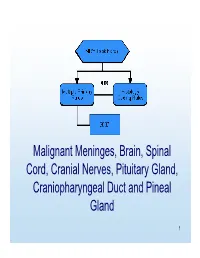
MP/H Rules Presentation
Malignant Meninges, Brain, Spinal Cord, Cranial Nerves, Pituitary Gland, Craniopharyngeal Duct and Pineal Gland 1 Equivalent Terms, Definitions, Charts and Illustrations • Benign and borderline intracranial and CNS tumors have a separate set of rules. 2 Equivalent Terms, Definitions, Charts and Illustrations • PNET (Primitive neuroectodermal tumor) – Central PNET – Supratentorial PNET • pPNET –not brain primary 3 Chart 1 Neuroepithelial Brain CNS • WHO Classification of Tumors of the brain and central nervous system • Not complete listing 4 Chart Instructions: Use this chart to code histology. The tree is arranged Neuroepithelial in descending order. Each branch is a histology group, starting at the top (9503) with the least specific terms and descending into more specific terms. Embryonal Ependymal Pineal Choroid plexus Neuronal and mixed Neuroblastic Glial Oligodendroglial tumors tumors tumors tumors neuronal-glial tumors tumors tumors tumors Ependymoma, Pineoblastoma Choroid plexus Olfactory neuroblastoma Oligodendroglioma NOS (9391) (9362) carcinoma Ganglioglioma, anaplastic (9522) NOS (9450) (9390) (9505 Olfactory neurocytoma Oligodendroglioma Ganglioglioma, malignant (9521) anaplastic (9451) Anasplastic ependymoma (9505) Olfactory neuroepithlioma Oligodendroblastoma (9392) (9523) (9460) Papillary ependymoma (9393) Glioma, NOS (9380) Atypical Ependymoblastoma Medulloepithelioma Medulloblastoma Supratentorial primitive tetratoid/rhabdoid (9392) (9501) (9470) neuroectodermal tumor tumor (9508) (PNET) (9473) Teratoid Demoplastic (9471) -

Mice Expressing Myc in Neural Precursors Develop Choroid Plexus and Ciliary Body Tumors
The American Journal of Pathology, Vol. 188, No. 6, June 2018 ajp.amjpathol.org ANIMAL MODELS Mice Expressing Myc in Neural Precursors Develop Choroid Plexus and Ciliary Body Tumors Morgan L. Shannon,* Ryann M. Fame,* Kevin F. Chau,*z Neil Dani,* Monica L. Calicchio,* Gwenaelle S. Géléoc,x{ Hart G.W. Lidov,* Sanda Alexandrescu,* and Maria K. Lehtinen*z From the Departments of Pathology* and Otolaryngologyx and the F.M. Kirby Center for Neurobiology,{ Boston Children’s Hospital, Boston; and the Program in Biological and Biomedical Sciences,z Harvard Medical School, Boston, Massachusetts Accepted for publication February 20, 2018. Choroid plexus tumors and ciliary body medulloepithelioma are predominantly pediatric neoplasms. Progress in understanding the pathogenesis of these tumors has been hindered by their rarity and lack Address correspondence to fi Maria K. Lehtinen, Ph.D., of models that faithfully recapitulate the disease. Here, we nd that endogenous Myc proto-oncogene Department of Pathology, Boston protein is down-regulated in the forebrain neuroepithelium, whose neural plate border domains give Children’s Hospital, 300 Long- rise to the anterior choroid plexus and ciliary body. To uncover the consequences of persistent Myc wood Ave., Boston, MA 02115. expression, MYC expression was forced in multipotent neural precursors (nestin-Cre:Myc), which E-mail: maria.lehtinen@ produced fully penetrant models of choroid plexus carcinoma and ciliary body medulloepithelioma. childrens.harvard.edu. Nestin-mediated MYC expression in the epithelial cells of choroid plexus leads to the regionalized formation of choroid plexus carcinoma in the posterior domain of the lateral ventricle choroid plexus and the fourth ventricle choroid plexus that is accompanied by loss of multiple cilia, up-regulation of protein biosynthetic machinery, and hydrocephalus. -

January 2020
Fred Hutch Cancer Surveillance System January, 2020 REGISTRAR PIP Visit SEER*Educate: A comprehensive training platform for registry professionals Clinical Grade for CNS Primaries Using the WHO Grading System for Selected Tumors of the CNS Mary Trimble, CTR If we have a diagnostic biopsy of a tumor, we can code the clinical grade regardless of the primary site. What is odd about Central Nervous System (CNS) tumors (Figures 1 and 2) is that we can code a clinical grade in the absence of a diagnostic biopsy or histologic confirmation for these primaries. For any other primary, we must have histologic or cytologic confirmation in order to code grade at all. So, what makes the Central Nervous System different? How is it we are able to code clinical grade without any histologic confirmation of the tumor? The World Health Organization (WHO) Classification of Tumors of the Central Nervous System, 4th Edition, released in 2016, provides the WHO grading system of CNS tumors as a uniform way to categorize CNS tumors. Figure 1 Figure 2 Lobes of the Cerebellum Pituitary and Pineal Glands American Joint CommiAee on Cancer (AJCC) Manual, 8th EdiFon - Table 72.2 This four-tiered grade scale helps to classify select CNS histologies based on proliferative potential and the risk of spread or recurrence. In other words, the WHO grade is a malignancy scale where the higher the WHO grade, the more aggressive the clinical course expected for the tumor.” This WHO classification can be seen as part of a larger clinical definition for select histologies and can therefore be used to assign grade without histologic confirmation. -

Primary Cerebral Neuroblastoma: CT and MR Findings in 12 Cases
115 Primary Cerebral Neuroblastoma: CT and MR Findings in 12 Cases P. C. Davis1 A retrospective CT, MR, and clinical study was performed in 12 patients, five children R. D. Wichman and seven adults, with histologically proved primary CNS neuroblastoma. The CT and Y. Takei MR appearances of this neoplasia were more variable than generally recognized. J. C. Hoffman, Jr. Although seven tumors were predominantly intraparenchymal masses with calcification and cyst formation, five were intra- or juxtaventricular. CT was preferable to noncontrast MR both at initial diagnosis and follow-up for identification of calcification, recurrent tumor at surgical sites, and leptomeningeal disease. Noncontrast MR was useful pri marily for localization of peri- and intraventricular lesions. We conclude that primary CNS neuroblastoma has a more variable radiographic appearance than is generally recognized, and that an intra- or periventricular epicenter is common. AJNR 11:115-120, January/February 1990; AJR 154: April1990 Primary cerebral neuroblastoma is a rare neoplasm typically described in children as a large intraparenchymal supratentorial mass frequently containing cysts, calci fication , and spontaneous hemorrhage [1-1 0]. This tumor is generally considered to be a specific subset of primitive neuroectodermal tumors, although neuropatho logic controversy exists. Detailed electron microscopy may be required for the exact diagnosis. These tumors are malignant lesions with a high rate of recurrence after therapy and frequent subarachnoid metastases. Our experience indicates that primary CNS neuroblastoma has a broader spectrum in presentation, age of onset, location, and radiographic appearance than is generally recognized. We report our experience with primary cerebral neuroblastoma in 12 patients and describe its CT and MR characteristics with clinical, surgical , and histological correlation. -

Molecular Cytogenetic Analysis in the Study of Brain Tumors: Findings and Applications
Neurosurg Focus 19 (5):E1, 2005 Molecular cytogenetic analysis in the study of brain tumors: findings and applications JANE BAYANI, M.H.SC., AJAY PANDITA, D.V.M., PH.D., AND JEREMY A. SQUIRE, PH.D. Department of Applied Molecular Oncology, Ontario Cancer Institute, Princess Margaret Hospital, University Health Network; Arthur and Sonia Labatt Brain Tumor Research Centre, Hospital for Sick Children; and Departments of Laboratory Medicine and Pathobiology and Medical Biophysics, University of Toronto, Ontario, Canada Classic cytogenetics has evolved from black and white to technicolor images of chromosomes as a result of advances in fluorescence in situ hybridization (FISH) techniques, and is now called molecular cytogenetics. Improvements in the quality and diversity of probes suitable for FISH, coupled with advances in computerized image analysis, now permit the genome or tissue of interest to be analyzed in detail on a glass slide. It is evident that the growing list of options for cytogenetic analysis has improved the understanding of chromosomal changes in disease initiation, progression, and response to treatment. The contributions of classic and molecular cytogenetics to the study of brain tumors have pro- vided scientists and clinicians alike with new avenues for investigation. In this review the authors summarize the con- tributions of molecular cytogenetics to the study of brain tumors, encompassing the findings of classic cytogenetics, interphase- and metaphase-based FISH studies, spectral karyotyping, and metaphase- and array-based comparative genomic hybridization. In addition, this review also details the role of molecular cytogenetic techniques in other aspects of understanding the pathogenesis of brain tumors, including xenograft, cancer stem cell, and telomere length studies.