Metallic Phases and Siderophile Elements in Main Group Ureilites: Implications for Ureilite Petrogenesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SPECTRAL CHARACTERIZATION of the ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 and 8159. KJ Orr1, LV Forman1, GK Benedix1
Ninth International Conference on Mars 2019 (LPI Contrib. No. 2089) 6177.pdf SPECTRAL CHARACTERIZATION OF THE ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 AND 8159. K. J. Orr1, L. V. Forman1, G. K. Benedix1, M. J. Hackett2, V. E. Hamilton3, and A. R. Santos. 1Space Science and Technology Centre (SSTC), School of Earth and Planetary Sciences, Curtin University, Perth, Western Australia, Australia ([email protected]), 2School of Molecular and Life Sciences, Curtin University, Perth, Western Australia, Australia, 3Southwest Research Institute, 1050 Walnut St. #300, Boulder, CO 80302 USA. 4USRA, 7178 Columbia Gateway Dr., Columbia, MD 21046. Introduction: Thermal infrared (TIR) spectros- Ga), the oldest confirmed shergottites recovered so far copy is a powerful remote sensing tool used to unravel [5]. As the only shergottites of Early Amazonian to No- the surface compositions of a target body. This tech- achian in age, they provide an invaluable opportunity to nique has been widely used in space missions, because understanding Mars’ early history. of its ability to detect and determine modal mineralogy Methods: Both samples (~0.5g chips) were ac- of the surface geology. It has been instrumental in de- quired from UNM and were made into epoxy mounts. veloping our understanding of Mars, as the majority of NWA 8159 was analyzed with a Tescan Integrated Min- missions sent to Mars have included an infrared spec- eral Analyzer (TIMA) to determine modal mineral trometer. These spectrometers can operate either in the abundancies and produce high-resolution mineral maps. visible (VIS) to near-infrared (NIR) or in the mid-infra- NWA 8159 was also analyzed using EBSD to charac- red (MIR). -

Physical Properties of Martian Meteorites: Porosity and Density Measurements
Meteoritics & Planetary Science 42, Nr 12, 2043–2054 (2007) Abstract available online at http://meteoritics.org Physical properties of Martian meteorites: Porosity and density measurements Ian M. COULSON1, 2*, Martin BEECH3, and Wenshuang NIE3 1Solid Earth Studies Laboratory (SESL), Department of Geology, University of Regina, Regina, Saskatchewan S4S 0A2, Canada 2Institut für Geowissenschaften, Universität Tübingen, 72074 Tübingen, Germany 3Campion College, University of Regina, Regina, Saskatchewan S4S 0A2, Canada *Corresponding author. E-mail: [email protected] (Received 11 September 2006; revision accepted 06 June 2007) Abstract–Martian meteorites are fragments of the Martian crust. These samples represent igneous rocks, much like basalt. As such, many laboratory techniques designed for the study of Earth materials have been applied to these meteorites. Despite numerous studies of Martian meteorites, little data exists on their basic structural characteristics, such as porosity or density, information that is important in interpreting their origin, shock modification, and cosmic ray exposure history. Analysis of these meteorites provides both insight into the various lithologies present as well as the impact history of the planet’s surface. We present new data relating to the physical characteristics of twelve Martian meteorites. Porosity was determined via a combination of scanning electron microscope (SEM) imagery/image analysis and helium pycnometry, coupled with a modified Archimedean method for bulk density measurements. Our results show a range in porosity and density values and that porosity tends to increase toward the edge of the sample. Preliminary interpretation of the data demonstrates good agreement between porosity measured at 100× and 300× magnification for the shergottite group, while others exhibit more variability. -

Evidence from Polymict Ureilite Meteorites for a Disrupted and Re-Accreted Single Ureilite Parent Asteroid Gardened by Several Distinct Impactors
Available online at www.sciencedirect.com Geochimica et Cosmochimica Acta 72 (2008) 4825–4844 www.elsevier.com/locate/gca Evidence from polymict ureilite meteorites for a disrupted and re-accreted single ureilite parent asteroid gardened by several distinct impactors Hilary Downes a,b,*, David W. Mittlefehldt c, Noriko T. Kita d, John W. Valley d a School of Earth Sciences, Birkbeck University of London, Malet Street, London WC1E 7HX, UK b Lunar and Planetary Institute, 3600 Bay Area Boulevard, Houston, TX 77058, USA c Mail Code KR, NASA/Johnson Space Center, Houston, TX 77058, USA d Department of Geology and Geophysics, University of Wisconsin-Madison, 1215 W. Dayton St., Madison, WI 53706, USA Received 25 July 2007; accepted in revised form 24 June 2008; available online 17 July 2008 Abstract Ureilites are ultramafic achondrites that exhibit heterogeneity in mg# and oxygen isotope ratios between different meteor- ites. Polymict ureilites represent near-surface material of the ureilite parent asteroid(s). Electron microprobe analyses of >500 olivine and pyroxene clasts in several polymict ureilites reveal a statistically identical range of compositions to that shown by unbrecciated ureilites, suggesting derivation from a single parent asteroid. Many ureilitic clasts have identical compositions to the anomalously high Mn/Mg olivines and pyroxenes from the Hughes 009 unbrecciated ureilite (here termed the ‘‘Hughes cluster”). Some polymict samples also contain lithic clasts derived from oxidized impactors. The presence of several common distinctive lithologies within polymict ureilites is additional evidence that ureilites were derived from a single parent asteroid. In situ oxygen three isotope analyses were made on individual ureilite minerals and lithic clasts, using a secondary ion mass spectrometer (SIMS) with precision typically better than 0.2–0.4& (2SD) for d18O and d17O. -

Moore County Unbrecciated Cumulate Eucrite, 1.88Kg
Moore County Unbrecciated Cumulate Eucrite, 1.88 kg Seen to fall Figure 1a: The Moore County eucrite (fusion crust on left), from the collection of the North Carolina Museum of Natural Sciences. Scale from top to bottom of sample is ~ 5 cm. Photo courtesy of Chris Tacker. Introduction: The Moore County meteorite (Figures 1a,b,c) fell at 5:00 PM on April 21, 1913, on the farm of George C. Graves, located approximately three miles east of Carthage, Moore County, North Carolina (79o23’W, 35o25’N) (Henderson and Davis, 1936). A loud “rumbling and zooming” noise “with no distinct explosions” was first observed within a five or six mile radius of the fall, followed by a sighting of a red hot ball with a 15-foot trail of blue-black smoke; the meteorite itself landed within a few feet of a farmer, in a nearly-vertical (but slightly SW-sloping) hole in a freshly-plowed field (Henderson and Davis, 1936). Only one stone was recovered (Figure 1b), weighing approximately 1.88 kg (4 lbs. 2 oz.), with maximum dimensions approximately 15 cm x 10.5 cm x 8 cm (6 in x 4 3/16 in x 3 3/16 in) (Henderson and Davis, 1936). This stone was divided between the US National Museum (Smithsonian) in Washington, D.C., and the North Carolina State Museum in Raleigh, now the North Carolina Museum of Natural Sciences (Henderson and Davis, 1936), where the main fractions of the stone are still kept (0.9 kg at the USNM and ~0.56 kg at the NC Museum: Grady, 2000; Tacker, pers. -

JEM-EUSO: Meteor and Nuclearite Observations
Exp Astron DOI 10.1007/s10686-014-9375-4 ORIGINAL ARTICLE JEM-EUSO: Meteor and nuclearite observations M. Bertaina A. Cellino F. Ronga The JEM-EUSO· Collaboration· · Received: 22 August 2013 / Accepted: 24 February 2014 ©SpringerScience+BusinessMediaDordrecht2014 Abstract Meteor and fireball observations are key to the derivation of both the inven- tory and physical characterization of small solar system bodies orbiting in the vicinity of the Earth. For several decades, observation of these phenomena has only been possible via ground-based instruments. The proposed JEM-EUSO mission has the potential to become the first operational space-based platform to share this capabil- ity. In comparison to the observation of extremely energetic cosmic ray events, which is the primary objective of JEM-EUSO, meteor phenomena are very slow, since their typical speeds are of the order of a few tens of km/sec (whereas cosmic rays travel at light speed). The observing strategy developed to detect meteors may also be applied to the detection of nuclearites, which have higher velocities, a wider range of possible trajectories, but move well below the speed of light and can therefore be considered as slow events for JEM-EUSO. The possible detection of nuclearites greatly enhances the scientific rationale behind the JEM-EUSO mission. Keywords Meteors Nuclearites JEM-EUSO Space detectors · · · M. Bertaina (!) Dipartimento di Fisica, Universit`adiTorino,INFNTorino,viaP.Giuria1,10125Torino,Italy e-mail: [email protected] A. Cellino (!) INAF-Osservatorio Astrofisico di Torino, Strada Osservatorio 20, 10025 Pino Torinese (TO), Italy e-mail: [email protected] F. Ronga (!) Istituto Nazionale di Fisica Nucleare - Laboratori Nazionali di Frascati, Via E. -

Graphite-Based Geothermometry on Almahata Sitta Ureilitic Meteorites
minerals Article Graphite-Based Geothermometry on Almahata Sitta Ureilitic Meteorites Anna Barbaro 1,*, M. Chiara Domeneghetti 1, Cyrena A. Goodrich 2, Moreno Meneghetti 3 , Lucio Litti 3, Anna Maria Fioretti 4, Peter Jenniskens 5 , Muawia H. Shaddad 6 and Fabrizio Nestola 7,8 1 Department of Earth and Environmental Sciences, University of Pavia, 27100 Pavia, Italy; [email protected] 2 Lunar and Planetary Institute, Universities Space Research Association, Houston, TX 77058, USA; [email protected] 3 Department of Chemical Sciences, University of Padova, 35131 Padova, Italy; [email protected] (M.M.); [email protected] (L.L.) 4 Institute of Geosciences and Earth Resources, National Research Council, 35131 Padova, Italy; anna.fi[email protected] 5 SETI Institute, Mountain View, CA 94043, USA; [email protected] 6 Department of Physics and Astronomy, University of Khartoum, Khartoum 11111, Sudan; [email protected] 7 Department of Geosciences, University of Padova, 35131 Padova, Italy; [email protected] 8 Geoscience Institute, Goethe-University Frankfurt, 60323 Frankfurt, Germany * Correspondence: [email protected]; Tel.: +39-3491548631 Received: 13 October 2020; Accepted: 10 November 2020; Published: 12 November 2020 Abstract: The thermal history of carbon phases, including graphite and diamond, in the ureilite meteorites has implications for the formation, igneous evolution, and impact disruption of their parent body early in the history of the Solar System. Geothermometry data were obtained by micro-Raman spectroscopy on graphite in Almahata Sitta (AhS) ureilites AhS 72, AhS 209b and AhS A135A from the University of Khartoum collection. In these samples, graphite shows G-band peak centers between 1 1578 and 1585 cm− and the full width at half maximum values correspond to a crystallization temperature of 1266 ◦C for graphite for AhS 209b, 1242 ◦C for AhS 72, and 1332 ◦C for AhS A135A. -

The Investigated Rock Is One of the Gabbroic Bodies Which Crops out in the Surroundings of Lyngdal ( Extreme South of Norway)
PYROXENE RELATIONS IN A HYPERITE NEAR LYNGDAL, NORWAY JOHAN J. LAVREAU Lavreau, J. J.: Pyroxene relations in a hyperite near Lyngdal, Norway. Norsk Geologisk Tidsskrift, Vol. 50, pp. 333-340. Oslo 1970. Pyroxenes from a hyperite body associated with Precambrian gneisses were studied optically, chemically, and by means of X-rays. Pyroxene relations show that crystallization takes place at a temperature higher than the inversion of pigeonite, and proceeds into the stability field of hypersthene. The composition and the mutual relations between the pyroxenes suggest a crystallization process for these minerals. Johan J. Lavreau, Laboratoire de Mineralogie et Petrologie, Universite Libre de Bruxelles, Brussels, Belgium. Introduction The investigated rock is one of the gabbroic bodies which crops out in the surroundings of Lyngdal ( extreme South of Norway). It Iies 3 km north of the town, northwest of Skoland lake, and is crossed by the E 18 road to Flekkefjord (Fig. 1). It is a well defined and homogeneous crescent-shaped unit, about 1500 m long and 500 m broad. The rock is medium grained (1-2 mm grain size), dark grey in colour, and has a gabbro-dioritic compo sition (Niggli 1923, p. 126). Some dioritic varieties were also recognized (Table 1). The country rock is a monzonitic phenoblastic gneiss foliated parallel to the elongation of the hyperite body. The contacts are seldom visible, for the area is covered by recent deposits except in its northern part, where the rock is unfortunately tectonized and strongly retrometa morphosed. Experimental techniques Plagioclase and pyroxenes were first studied with the aid of the universal stage. -

Impact Shock Origin of Diamonds in Ureilite Meteorites
Impact shock origin of diamonds in ureilite meteorites Fabrizio Nestolaa,b,1, Cyrena A. Goodrichc,1, Marta Moranad, Anna Barbarod, Ryan S. Jakubeke, Oliver Christa, Frank E. Brenkerb, M. Chiara Domeneghettid, M. Chiara Dalconia, Matteo Alvarod, Anna M. Fiorettif, Konstantin D. Litasovg, Marc D. Friesh, Matteo Leonii,j, Nicola P. M. Casatik, Peter Jenniskensl, and Muawia H. Shaddadm aDepartment of Geosciences, University of Padova, I-35131 Padova, Italy; bGeoscience Institute, Goethe University Frankfurt, 60323 Frankfurt, Germany; cLunar and Planetary Institute, Universities Space Research Association, Houston, TX 77058; dDepartment of Earth and Environmental Sciences, University of Pavia, I-27100 Pavia, Italy; eAstromaterials Research and Exploration Science Division, Jacobs Johnson Space Center Engineering, Technology and Science, NASA, Houston, TX 77058; fInstitute of Geosciences and Earth Resources, National Research Council, I-35131 Padova, Italy; gVereshchagin Institute for High Pressure Physics RAS, Troitsk, 108840 Moscow, Russia; hNASA Astromaterials Acquisition and Curation Office, Johnson Space Center, NASA, Houston, TX 77058; iDepartment of Civil, Environmental and Mechanical Engineering, University of Trento, I-38123 Trento, Italy; jSaudi Aramco R&D Center, 31311 Dhahran, Saudi Arabia; kSwiss Light Source, Paul Scherrer Institut, 5232 Villigen, Switzerland; lCarl Sagan Center, SETI Institute, Mountain View, CA 94043; and mDepartment of Physics and Astronomy, University of Khartoum, 11111 Khartoum, Sudan Edited by Mark Thiemens, University of California San Diego, La Jolla, CA, and approved August 12, 2020 (received for review October 31, 2019) The origin of diamonds in ureilite meteorites is a timely topic in to various degrees and in these samples the graphite areas, though planetary geology as recent studies have proposed their formation still having external blade-shaped morphologies, are internally at static pressures >20 GPa in a large planetary body, like diamonds polycrystalline (18). -

Pigeonite Basalt 2109 Grams
12065 DRAFT Pigeonite Basalt 2109 grams Figure 1: Photo of 12065 showing numerous zap pits on rounded surface. Scale is in cm. NASA # S69-60591. Introduction Sample 12065 is a large rounded pigeonite basalt dated that near-surface olivine (Fo74) and some pyroxene at 3.16 ± 0.09 b.y. The outer surface is covered with settling could explain the variation in composition of micrometeorite pits on all sides (figure 1). some Apollo 12 basalts. Petrography Mineralogy 12065 is a variolitic basalt composed of pyroxene and Olivine: Olivine composition in 12065 ranges from olivine phenocrysts (figure 2) imbedded in a very fine Fo72-32 (Kushiro et al. 1971). matrix of feathery ilmenite, plagioclase and clinopyroxene (figure 3)(Reid 1971). Kushiro et al. Pyroxene: Hollister et al. (1971) and Kushiro et al. (1971) find that the fibrous pyroxene in 12065 is similar (1971) describe complex sector zoning of pyroxene to “quench pyroxenes” often found in quenching phenocrysts in 12065 (figure 4). Pigeonite cores are experiments. 12065 has a few percent void space. overgrown by subcalcic augite (Gay et al. 1971). Kushiro et al. report extreme Fe-enrichment in matrix Kushiro et al. (1971) used the bulk composition of pyroxene. Gay et al. report pyroxferroite with low Ca. 12065 to perform experiments leading to the conclusion Lunar Sample Compendium C Meyer 2005 Figure 2: Photomicrograph of thin section 12065 showing elongate pyroxene in variolitic groundmass. Scale is about 2 cm. NASA # S69-23378. Plagioclase: Plagioclase is An91 – An89 (Kushiro et al. 1971). Chemistry The chemical composition of 12065 has been reported Spinel: The Ti content of the Cr-spinel increases with by LSPET (1970), Maxwell et al. -

2713^7 Contents
MINERALS OF WASHINGTON, D.C. AND VICINITY by Lawrence R. Bernstein U. S. Geo^r^'ce.l Survey OPEN F-'::. r;.".r'0.?;r cer.-..: 2713^7 CONTENTS Introduction 1 Scope of report 4 Mineral collecting 5 Acknowledgments 6 Introduction 6a ITT3 Aclj.il1 -> ! T^______.___~^. -"» _«_____«..«_»«__.. " " .._.«__._.._*_.__._.,_.._.-. _>-.-- -_>-->.-..-. Q Triassic deposits 31 Mineral localities 38 District of Columbia : 38 IMavtrlWlCfci JT XClilUl a Tirl ~ __ ___« - - -_ -»-i-___ .__- _ __- - ________________ m~m~m~ m~ m~ «M » M* **A^J ^ Anne Arundel County 43 Baltimore County 45 Howard County - 74 Montgomery County 88 Prince Georges County 120 Virginia . 129 Arlington County 129 Fairfax County 131 Fauquier County 139 Loudoun County 143 Prince William County 149 Diabase quarries of northern Virginia 155 CAPTIONS Illustrations Plate 1. Mineral localities of Washington, B.C., and vicinity. Plate 2. Generalized geologic map of Washington. D.C. and vicinity, Plate 3. Mineral deposits and generalized geology of the Triassic rocks near Washington, D.C. List of Figures Figure 1. Index map showing region covered in this report. Stfaded area is covered in most detail. Figure 2. Block diagram of the Washington, D.C. region showing physiographic provinces and major geographic and geologic features. ITfgure -3. Coastal Plain deposits of Washington, D.C. and vicinity. Figure 4. Generalized cross section of a typical complex pegmatite of the Washington, D.C. area. Figure 5. Rhythmically.layered gabbro of the Baltimore Gabbro Com plex at Ilchester, Maryland. Figure 6. Triassic diabase dike forming a ridge north of Route 7 near Dranesville, Virginia. -
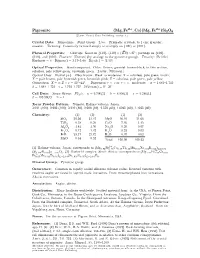
Pigeonite (Mg; Fe ; Ca)(Mg; Fe )Si2o6 C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Monoclinic
2+ 2+ Pigeonite (Mg; Fe ; Ca)(Mg; Fe )Si2O6 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Prismatic crystals, to 1 cm; granular, massive. Twinning: Commonly twinned simply or multiply on 100 or 001 . f g f g Physical Properties: Cleavage: Good on 110 , (110) (110) 87 ; partings on 100 , f g ^ » ± f g 010 , and 001 . Fracture: [Uneven] (by analogy to the pyroxene group). Tenacity: [Brittle.] Hf ardgness =f6 Dg (meas.) = 3.17{3.46 D(calc.) = [3.53] Optical Properties: Semitransparent. Color: Brown, greenish brown-black; in thin section, colorless, pale yellow-green, brownish green. Luster: [Vitreous.] Optical Class: Biaxial (+). Pleochroism: Weak to moderate; X = colorless, pale green, brown; Y = pale brown, pale brownish green, brownish pink; Z = colorless, pale green, pale yellow. Orientation: X = a; Z c = 32 {44 . Dispersion: r < v or r > v; moderate. ® = 1.682{1.732 ^ ± ± ¯ = 1.684{1.732 ° = 1.705{1.757 2V(meas.) = 0±{30± Cell Data: Space Group: P 21=c: a = 9.706(2) b = 8.950(1) c = 5.246(1) ¯ = 108:59(1)± Z = 4 X-ray Powder Pattern: Yumoto, Hakone volcano, Japan. 3.021 (100), 2.903 (100), 3.210 (80), 2.908 (80), 2.578 (60), 1.6265 (60), 1.4935 (60) Chemistry: (1) (2) (1) (2) SiO2 50.56 51.47 MgO 16.10 21.68 TiO2 0.58 0.29 CaO 7.05 1.45 Al2O3 1.41 1.56 Na2O 0.26 0.07 Fe2O3 0.12 1.42 K2O 0.23 0.03 FeO 23.17 21.72 H2O¡ 0.07 0.02 MnO 0.54 0.52 Total 100.09 100.23 2+ (1) Hakone volcano, Japan; corresponds to (Mg0:92Fe0:73Ca0:29Ti0:02Mn0:02Na0:02K0:01)§=2:01 2+ (Si1:94Al0:06)§=2:00O6: (2) Bushveld complex, South Africa; corresponds to (Mg1:21Fe0:68Ca0:06 3+ Fe0:03Mn0:02Ti0:01)§=2:01(Si1:93Al0:07)§=2:00O6: Mineral Group: Pyroxene group. -

Plagioclase-Bearing Monomict Ureilite Or Ungrouped Achondrite?
Meteoritics & Planetary Science 41, Nr 6, 925–952 (2006) Abstract available online at http://meteoritics.org Northwest Africa 1500: Plagioclase-bearing monomict ureilite or ungrouped achondrite? Cyrena Anne GOODRICH1*, Frank WLOTZKA2, D. Kent ROSS3, and Rainer BARTOSCHEWITZ4 1Department of Physical Sciences, Kingsborough Community College, 2001 Oriental Boulevard, Brooklyn, New York 11235, USA 2Max Planck Institute for Chemistry, PO 3060, D-55020 Mainz, Germany 3School of Ocean and Earth Sciences and Technology, University of Hawai’i at Manoa, Honolulu, Hawai’i 96822, USA 4Meteorite Laboratory Lehmweg 53 D-38518 Gifhorn, Germany *Corresponding author. E-mail: [email protected] (Received 03 October 2005; revision accepted 03 March 2006) Abstract–Northwest Africa (NWA) 1500 is an ultramafic meteorite dominated by coarse (∼100– 500 μm) olivine (95–96%), augite (2–3%), and chromite (0.6–1.6%) in an equilibrated texture. Plagioclase (0.7–1.8%) occurs as poikilitic grains (up to ∼3 mm) in vein-like areas that have concentrations of augite and minor orthopyroxene. Other phases are Cl-apatite, metal, sulfide, and graphite. Olivine ranges from Fo 65–73, with a strong peak at Fo 68–69. Most grains are reverse- zoned, and also have ∼10–30 μm reduction rims. In terms of its dominant mineralogy and texture, NWA 1500 resembles the majority of monomict ureilites. However, it is more ferroan than known ureilites (Fo ≥75) and other mineral compositional parameters are out of the ureilite range as well. Furthermore, neither apatite nor plagioclase have ever been observed, and chromite is rare in monomict ureilites. Nevertheless, this meteorite may be petrologically related to the rare augite-bearing ureilites and represent a previously unsampled part of the ureilite parent body (UPB).