Ca–Mg Diffusion in Diopside: Tracer and Chemical Inter-Diffusion Coefficients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Module 7 Igneous Rocks IGNEOUS ROCKS
Module 7 Igneous Rocks IGNEOUS ROCKS ▪ Igneous Rocks form by crystallization of molten rock material IGNEOUS ROCKS ▪ Igneous Rocks form by crystallization of molten rock material ▪ Molten rock material below Earth’s surface is called magma ▪ Molten rock material erupted above Earth’s surface is called lava ▪ The name changes because the composition of the molten material changes as it is erupted due to escape of volatile gases Rocks Cycle Consolidation Crystallization Rock Forming Minerals 1200ºC Olivine High Ca-rich Pyroxene Ca-Na-rich Amphibole Intermediate Na-Ca-rich Continuous branch Continuous Discontinuous branch Discontinuous Biotite Na-rich Plagioclase feldspar of liquid increases liquid of 2 Temperature decreases Temperature SiO Low K-feldspar Muscovite Quartz 700ºC BOWEN’S REACTION SERIES Rock Forming Minerals Olivine Ca-rich Pyroxene Ca-Na-rich Amphibole Na-Ca-rich Continuous branch Continuous Discontinuous branch Discontinuous Biotite Na-rich Plagioclase feldspar K-feldspar Muscovite Quartz BOWEN’S REACTION SERIES Rock Forming Minerals High Temperature Mineral Suite Olivine • Isolated Tetrahedra Structure • Iron, magnesium, silicon, oxygen • Bowen’s Discontinuous Series Augite • Single Chain Structure (Pyroxene) • Iron, magnesium, calcium, silicon, aluminium, oxygen • Bowen’s Discontinuos Series Calcium Feldspar • Framework Silicate Structure (Plagioclase) • Calcium, silicon, aluminium, oxygen • Bowen’s Continuous Series Rock Forming Minerals Intermediate Temperature Mineral Suite Hornblende • Double Chain Structure (Amphibole) -

Mid-Infrared Optical Constants of Clinopyroxene and Orthoclase Derived from Oriented Single-Crystal Reflectance Spectra
American Mineralogist, Volume 99, pages 1942–1955, 2014 Mid-infrared optical constants of clinopyroxene and orthoclase derived from oriented single-crystal reflectance spectra JESSICA A. ARNOLD1,*, TIMOTHY D. GLOTCH1 AND ANNA M. PLONKA1 1Department of Geosciences, Stony Brook University, Stony Brook, New York 11794, U.S.A. ABSTRACT We have determined the mid-IR optical constants of one alkali feldspar and four pyroxene compo- sitions in the range of 250–4000 cm–1. Measured reflectance spectra of oriented single crystals were iteratively fit to modeled spectra derived from classical dispersion analysis. We present the real and imaginary indices of refraction (n and k) along with the oscillator parameters with which they were modeled. While materials of orthorhombic symmetry and higher are well covered by the current literature, optical constants have been derived for only a handful of geologically relevant monoclinic materials, including gypsum and orthoclase. Two input parameters that go into radiative transfer models, the scattering phase function and the single scattering albedo, are functions of a material’s optical constants. Pyroxene is a common rock-forming mineral group in terrestrial bodies as well as meteorites and is also detected in cosmic dust. Hence, having a set of pyroxene optical constants will provide additional details about the composition of Solar System bodies and circumstellar materials. We follow the method of Mayerhöfer et al. (2010), which is based on the Berreman 4 × 4 matrix formulation. This approach provides a consistent way to calculate the reflectance coefficients in low- symmetry cases. Additionally, while many models assume normal incidence to simplify the dispersion relations, this more general model applies to reflectance spectra collected at non-normal incidence. -

SPECTRAL CHARACTERIZATION of the ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 and 8159. KJ Orr1, LV Forman1, GK Benedix1
Ninth International Conference on Mars 2019 (LPI Contrib. No. 2089) 6177.pdf SPECTRAL CHARACTERIZATION OF THE ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 AND 8159. K. J. Orr1, L. V. Forman1, G. K. Benedix1, M. J. Hackett2, V. E. Hamilton3, and A. R. Santos. 1Space Science and Technology Centre (SSTC), School of Earth and Planetary Sciences, Curtin University, Perth, Western Australia, Australia ([email protected]), 2School of Molecular and Life Sciences, Curtin University, Perth, Western Australia, Australia, 3Southwest Research Institute, 1050 Walnut St. #300, Boulder, CO 80302 USA. 4USRA, 7178 Columbia Gateway Dr., Columbia, MD 21046. Introduction: Thermal infrared (TIR) spectros- Ga), the oldest confirmed shergottites recovered so far copy is a powerful remote sensing tool used to unravel [5]. As the only shergottites of Early Amazonian to No- the surface compositions of a target body. This tech- achian in age, they provide an invaluable opportunity to nique has been widely used in space missions, because understanding Mars’ early history. of its ability to detect and determine modal mineralogy Methods: Both samples (~0.5g chips) were ac- of the surface geology. It has been instrumental in de- quired from UNM and were made into epoxy mounts. veloping our understanding of Mars, as the majority of NWA 8159 was analyzed with a Tescan Integrated Min- missions sent to Mars have included an infrared spec- eral Analyzer (TIMA) to determine modal mineral trometer. These spectrometers can operate either in the abundancies and produce high-resolution mineral maps. visible (VIS) to near-infrared (NIR) or in the mid-infra- NWA 8159 was also analyzed using EBSD to charac- red (MIR). -

Moore County Unbrecciated Cumulate Eucrite, 1.88Kg
Moore County Unbrecciated Cumulate Eucrite, 1.88 kg Seen to fall Figure 1a: The Moore County eucrite (fusion crust on left), from the collection of the North Carolina Museum of Natural Sciences. Scale from top to bottom of sample is ~ 5 cm. Photo courtesy of Chris Tacker. Introduction: The Moore County meteorite (Figures 1a,b,c) fell at 5:00 PM on April 21, 1913, on the farm of George C. Graves, located approximately three miles east of Carthage, Moore County, North Carolina (79o23’W, 35o25’N) (Henderson and Davis, 1936). A loud “rumbling and zooming” noise “with no distinct explosions” was first observed within a five or six mile radius of the fall, followed by a sighting of a red hot ball with a 15-foot trail of blue-black smoke; the meteorite itself landed within a few feet of a farmer, in a nearly-vertical (but slightly SW-sloping) hole in a freshly-plowed field (Henderson and Davis, 1936). Only one stone was recovered (Figure 1b), weighing approximately 1.88 kg (4 lbs. 2 oz.), with maximum dimensions approximately 15 cm x 10.5 cm x 8 cm (6 in x 4 3/16 in x 3 3/16 in) (Henderson and Davis, 1936). This stone was divided between the US National Museum (Smithsonian) in Washington, D.C., and the North Carolina State Museum in Raleigh, now the North Carolina Museum of Natural Sciences (Henderson and Davis, 1936), where the main fractions of the stone are still kept (0.9 kg at the USNM and ~0.56 kg at the NC Museum: Grady, 2000; Tacker, pers. -

The Investigated Rock Is One of the Gabbroic Bodies Which Crops out in the Surroundings of Lyngdal ( Extreme South of Norway)
PYROXENE RELATIONS IN A HYPERITE NEAR LYNGDAL, NORWAY JOHAN J. LAVREAU Lavreau, J. J.: Pyroxene relations in a hyperite near Lyngdal, Norway. Norsk Geologisk Tidsskrift, Vol. 50, pp. 333-340. Oslo 1970. Pyroxenes from a hyperite body associated with Precambrian gneisses were studied optically, chemically, and by means of X-rays. Pyroxene relations show that crystallization takes place at a temperature higher than the inversion of pigeonite, and proceeds into the stability field of hypersthene. The composition and the mutual relations between the pyroxenes suggest a crystallization process for these minerals. Johan J. Lavreau, Laboratoire de Mineralogie et Petrologie, Universite Libre de Bruxelles, Brussels, Belgium. Introduction The investigated rock is one of the gabbroic bodies which crops out in the surroundings of Lyngdal ( extreme South of Norway). It Iies 3 km north of the town, northwest of Skoland lake, and is crossed by the E 18 road to Flekkefjord (Fig. 1). It is a well defined and homogeneous crescent-shaped unit, about 1500 m long and 500 m broad. The rock is medium grained (1-2 mm grain size), dark grey in colour, and has a gabbro-dioritic compo sition (Niggli 1923, p. 126). Some dioritic varieties were also recognized (Table 1). The country rock is a monzonitic phenoblastic gneiss foliated parallel to the elongation of the hyperite body. The contacts are seldom visible, for the area is covered by recent deposits except in its northern part, where the rock is unfortunately tectonized and strongly retrometa morphosed. Experimental techniques Plagioclase and pyroxenes were first studied with the aid of the universal stage. -

Pigeonite Basalt 2109 Grams
12065 DRAFT Pigeonite Basalt 2109 grams Figure 1: Photo of 12065 showing numerous zap pits on rounded surface. Scale is in cm. NASA # S69-60591. Introduction Sample 12065 is a large rounded pigeonite basalt dated that near-surface olivine (Fo74) and some pyroxene at 3.16 ± 0.09 b.y. The outer surface is covered with settling could explain the variation in composition of micrometeorite pits on all sides (figure 1). some Apollo 12 basalts. Petrography Mineralogy 12065 is a variolitic basalt composed of pyroxene and Olivine: Olivine composition in 12065 ranges from olivine phenocrysts (figure 2) imbedded in a very fine Fo72-32 (Kushiro et al. 1971). matrix of feathery ilmenite, plagioclase and clinopyroxene (figure 3)(Reid 1971). Kushiro et al. Pyroxene: Hollister et al. (1971) and Kushiro et al. (1971) find that the fibrous pyroxene in 12065 is similar (1971) describe complex sector zoning of pyroxene to “quench pyroxenes” often found in quenching phenocrysts in 12065 (figure 4). Pigeonite cores are experiments. 12065 has a few percent void space. overgrown by subcalcic augite (Gay et al. 1971). Kushiro et al. report extreme Fe-enrichment in matrix Kushiro et al. (1971) used the bulk composition of pyroxene. Gay et al. report pyroxferroite with low Ca. 12065 to perform experiments leading to the conclusion Lunar Sample Compendium C Meyer 2005 Figure 2: Photomicrograph of thin section 12065 showing elongate pyroxene in variolitic groundmass. Scale is about 2 cm. NASA # S69-23378. Plagioclase: Plagioclase is An91 – An89 (Kushiro et al. 1971). Chemistry The chemical composition of 12065 has been reported Spinel: The Ti content of the Cr-spinel increases with by LSPET (1970), Maxwell et al. -

Mineral Collecting Sites in North Carolina by W
.'.' .., Mineral Collecting Sites in North Carolina By W. F. Wilson and B. J. McKenzie RUTILE GUMMITE IN GARNET RUBY CORUNDUM GOLD TORBERNITE GARNET IN MICA ANATASE RUTILE AJTUNITE AND TORBERNITE THULITE AND PYRITE MONAZITE EMERALD CUPRITE SMOKY QUARTZ ZIRCON TORBERNITE ~/ UBRAR'l USE ONLV ,~O NOT REMOVE. fROM LIBRARY N. C. GEOLOGICAL SUHVEY Information Circular 24 Mineral Collecting Sites in North Carolina By W. F. Wilson and B. J. McKenzie Raleigh 1978 Second Printing 1980. Additional copies of this publication may be obtained from: North CarOlina Department of Natural Resources and Community Development Geological Survey Section P. O. Box 27687 ~ Raleigh. N. C. 27611 1823 --~- GEOLOGICAL SURVEY SECTION The Geological Survey Section shall, by law"...make such exami nation, survey, and mapping of the geology, mineralogy, and topo graphy of the state, including their industrial and economic utilization as it may consider necessary." In carrying out its duties under this law, the section promotes the wise conservation and use of mineral resources by industry, commerce, agriculture, and other governmental agencies for the general welfare of the citizens of North Carolina. The Section conducts a number of basic and applied research projects in environmental resource planning, mineral resource explora tion, mineral statistics, and systematic geologic mapping. Services constitute a major portion ofthe Sections's activities and include identi fying rock and mineral samples submitted by the citizens of the state and providing consulting services and specially prepared reports to other agencies that require geological information. The Geological Survey Section publishes results of research in a series of Bulletins, Economic Papers, Information Circulars, Educa tional Series, Geologic Maps, and Special Publications. -

PRELIMINARY EVALUATION of BEDROCK POTENTIAL for NATURALLY OCCURRING ASBESTOS in ALASKA by Diana N
Alaska Division of Geological & Geophysical Surveys MISCELLANEOUS PUBLICATION 157 PRELIMINARY EVALUATION OF BEDROCK POTENTIAL FOR NATURALLY OCCURRING ASBESTOS IN ALASKA by Diana N. Solie and Jennifer E. Athey Tremolite (UAMES 34960) displaying the soft, friable fibers of asbestiform minerals. Sample collected from the Cosmos Hills area, Kobuk District, Alaska, by Eskil Anderson. Image courtesy of the University of Alaska Museum Earth Sciences Department. June 2015 Released by STATE OF ALASKA DEPARTMENT OF NATURAL RESOURCES Division of Geological & Geophysical Surveys 3354 College Road, Fairbanks, Alaska 99709-3707 907-451-5020 dggs.alaska.gov [email protected] $2.00 (text only) $13.00 (per map sheet) TABLE OF CONTENTS Abstract ................................................................................................................................................................................................................................. 1 Introduction ........................................................................................................................................................................................................................ 1 General geology of asbestos ......................................................................................................................................................................................... 2 Naturally occurring asbestos potential in Alaska .............................................................................................................................................. -

2713^7 Contents
MINERALS OF WASHINGTON, D.C. AND VICINITY by Lawrence R. Bernstein U. S. Geo^r^'ce.l Survey OPEN F-'::. r;.".r'0.?;r cer.-..: 2713^7 CONTENTS Introduction 1 Scope of report 4 Mineral collecting 5 Acknowledgments 6 Introduction 6a ITT3 Aclj.il1 -> ! T^______.___~^. -"» _«_____«..«_»«__.. " " .._.«__._.._*_.__._.,_.._.-. _>-.-- -_>-->.-..-. Q Triassic deposits 31 Mineral localities 38 District of Columbia : 38 IMavtrlWlCfci JT XClilUl a Tirl ~ __ ___« - - -_ -»-i-___ .__- _ __- - ________________ m~m~m~ m~ m~ «M » M* **A^J ^ Anne Arundel County 43 Baltimore County 45 Howard County - 74 Montgomery County 88 Prince Georges County 120 Virginia . 129 Arlington County 129 Fairfax County 131 Fauquier County 139 Loudoun County 143 Prince William County 149 Diabase quarries of northern Virginia 155 CAPTIONS Illustrations Plate 1. Mineral localities of Washington, B.C., and vicinity. Plate 2. Generalized geologic map of Washington. D.C. and vicinity, Plate 3. Mineral deposits and generalized geology of the Triassic rocks near Washington, D.C. List of Figures Figure 1. Index map showing region covered in this report. Stfaded area is covered in most detail. Figure 2. Block diagram of the Washington, D.C. region showing physiographic provinces and major geographic and geologic features. ITfgure -3. Coastal Plain deposits of Washington, D.C. and vicinity. Figure 4. Generalized cross section of a typical complex pegmatite of the Washington, D.C. area. Figure 5. Rhythmically.layered gabbro of the Baltimore Gabbro Com plex at Ilchester, Maryland. Figure 6. Triassic diabase dike forming a ridge north of Route 7 near Dranesville, Virginia. -
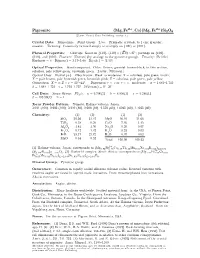
Pigeonite (Mg; Fe ; Ca)(Mg; Fe )Si2o6 C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Monoclinic
2+ 2+ Pigeonite (Mg; Fe ; Ca)(Mg; Fe )Si2O6 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Prismatic crystals, to 1 cm; granular, massive. Twinning: Commonly twinned simply or multiply on 100 or 001 . f g f g Physical Properties: Cleavage: Good on 110 , (110) (110) 87 ; partings on 100 , f g ^ » ± f g 010 , and 001 . Fracture: [Uneven] (by analogy to the pyroxene group). Tenacity: [Brittle.] Hf ardgness =f6 Dg (meas.) = 3.17{3.46 D(calc.) = [3.53] Optical Properties: Semitransparent. Color: Brown, greenish brown-black; in thin section, colorless, pale yellow-green, brownish green. Luster: [Vitreous.] Optical Class: Biaxial (+). Pleochroism: Weak to moderate; X = colorless, pale green, brown; Y = pale brown, pale brownish green, brownish pink; Z = colorless, pale green, pale yellow. Orientation: X = a; Z c = 32 {44 . Dispersion: r < v or r > v; moderate. ® = 1.682{1.732 ^ ± ± ¯ = 1.684{1.732 ° = 1.705{1.757 2V(meas.) = 0±{30± Cell Data: Space Group: P 21=c: a = 9.706(2) b = 8.950(1) c = 5.246(1) ¯ = 108:59(1)± Z = 4 X-ray Powder Pattern: Yumoto, Hakone volcano, Japan. 3.021 (100), 2.903 (100), 3.210 (80), 2.908 (80), 2.578 (60), 1.6265 (60), 1.4935 (60) Chemistry: (1) (2) (1) (2) SiO2 50.56 51.47 MgO 16.10 21.68 TiO2 0.58 0.29 CaO 7.05 1.45 Al2O3 1.41 1.56 Na2O 0.26 0.07 Fe2O3 0.12 1.42 K2O 0.23 0.03 FeO 23.17 21.72 H2O¡ 0.07 0.02 MnO 0.54 0.52 Total 100.09 100.23 2+ (1) Hakone volcano, Japan; corresponds to (Mg0:92Fe0:73Ca0:29Ti0:02Mn0:02Na0:02K0:01)§=2:01 2+ (Si1:94Al0:06)§=2:00O6: (2) Bushveld complex, South Africa; corresponds to (Mg1:21Fe0:68Ca0:06 3+ Fe0:03Mn0:02Ti0:01)§=2:01(Si1:93Al0:07)§=2:00O6: Mineral Group: Pyroxene group. -

Papers and Proceedings of the Royal Society of Tasmania
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Tasmania Open Access Repository ON MESOZOIC DOLERITP] AND DIABASE IN TASMANIA. By W. H. Twblvetrees, F.G.S., and W. F. Petterd, C.M.Z.S. The following Notes lay no claim to be an exhaustive description of our familiar '• diabase" or "dolerite" rock, which plays such an important part in the geology and physical configuration of our Island. The present object is rather to place upon record some inferences drawn from the examination of numerous microscopical sections of speci- mens collected or received from all parts of Tasmania. It is by accumulating the results of observations that stepping stones are formed to more complete knowledge. A glance at Mr. R. M. Johnston's geological map of Tasmania, issued by the Lands Office, will show the share this rock takes in the structure of the Island. It occupies the whole upland area of the Central Tiers. On the northern face of the Tiers—the Western Tiers as they are here called—there is a tongue of the rock prolonged northwards past IMount Claude. At their north-west corner it forms or caps mountains, such as Cradle Mountain (the highest in Tas- mania), Barn Bluff, Mount Pelion West. Eldon Blufi: forms a narrow western extension. Mount Sedgwick is a western out-lier ; Mount Dundas another. In that part of the island it is also found at Mount Heemskirk Falls, and on the Magnet Range, two miles north of the Magnet Mine. Mounts Gell and Hugel are also western out-liers. -

A Comparative Study of Jadeite, Omphacite and Kosmochlor Jades from Myanmar, and Suggestions for a Practical Nomenclature
Feature Article A Comparative Study of Jadeite, Omphacite and Kosmochlor Jades from Myanmar, and Suggestions for a Practical Nomenclature Leander Franz, Tay Thye Sun, Henry A. Hänni, Christian de Capitani, Theerapongs Thanasuthipitak and Wilawan Atichat Jadeitite boulders from north-central Myanmar show a wide variability in texture and mineral content. This study gives an overview of the petrography of these rocks, and classiies them into ive different types: (1) jadeitites with kosmochlor and clinoamphibole, (2) jadeitites with clinoamphibole, (3) albite-bearing jadeitites, (4) almost pure jadeitites and (5) omphacitites. Their textures indicate that some of the assemblages formed syn-tectonically while those samples with decussate textures show no indication of a tectonic overprint. Backscattered electron images and electron microprobe analyses highlight the variable mineral chemistry of the samples. Their extensive chemical and textural inhomogeneity renders a classiication by common gemmological methods rather dificult. Although a deinitive classiication of such rocks is only possible using thin-section analysis, we demonstrate that a fast and non-destructive identiication as jadeite jade, kosmochlor jade or omphacite jade is possible using Raman and infrared spectroscopy, which gave results that were in accord with the microprobe analyses. Furthermore, current classiication schemes for jadeitites are reviewed. The Journal of Gemmology, 34(3), 2014, pp. 210–229, http://dx.doi.org/10.15506/JoG.2014.34.3.210 © 2014 The Gemmological Association of Great Britain Introduction simple. Jadeite jade is usually a green massive The word jade is derived from the Spanish phrase rock consisting of jadeite (NaAlSi2O6; see Ou Yang, for piedra de ijada (Foshag, 1957) or ‘loin stone’ 1999; Ou Yang and Li, 1999; Ou Yang and Qi, from its reputed use in curing ailments of the loins 2001).