Nat's Offiicialfontthesisforms-1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Feasibility Analysis of Laundry Lounge in Vietnam, Targeting Students
FEASIBILITY ANALYSIS OF LAUNDRY LOUNGE IN VIETNAM, TARGETING STUDENTS Case: Start-up X, Ho Chi Minh, Vietnam LAHTI UNIVERSITY OF APPLIED SCIENCES Degree program in International Business Thesis Spring 2015 Nguyen, Thi Cam Tu Nguyen, Truong Minh Dang Lahti University of Applied Sciences Degree Programme in International Business NGUYEN, THI CAM TU & Feasibility analysis of laundry lounge in Vietnam, targeting students NGUYEN, TRUONG MINH DANG Case: Start-up X, Ho Chi Minh, Vietnam Bachelor’s Thesis in International Business, 111 pages, 4 pages of appendices Spring 2015 ABSTRACT This study aims to propose a business idea – a laundry lounge for students in Ho Chi Minh City, Vietnam. In order to achieve this aim, a feasibility analysis is applied to determine whether the laundry lounge, a combination of laundry and extra services such as cafeteria, reading room and free Wi-Fi, is promising for the target group. As a result, the objective of the thesis is to measure the feasibility of the laundry lounge model in the target market. The thesis is structured with four main parts. To begin with, the main theories in business idea evaluation are thoroughly mentioned. With its components, the feasibility analysis is employed throughout this study as the main metrics for the proposed business in order to measure its potentiality. Then, a description of laundry lounge concept and its basic form, self-service laundry, are clearly presented. After that, an empirical research to collect primary data regarding the laundry lounge concept is logically reported. Finally, the feasibility analysis is implemented in detail to find out the answers for the research questions and hence successfully serve the objective of this thesis. -

Toxin-Free Home Shopping Guide Ebook
WELCOME Welcome to my Toxin-Free Home Shopping Guide! This guide has some of our favorite products and recipes that we use in our homes. I’ve broken it out by room with links and descriptions. I even have a few helpful tips sprinkled in to make your journey to a toxin-free home easier! ~Aimee Carlson~ THE TOXIN TERMINATOR 01 KITCHEN 1. Young Living- www.aimeecarlson.com/start Thieves Products=Cleaners, Dish Soaps, Hand Soaps Diffusers and Essential Oils 2. My Green Fills http://click.mygreenfills.com/aff_c?offer_id=2&aff_id=2912 Cleaners, Dish Soaps 3. Berkey - Water Filtration Systems https://www.berkeyfilters.com/collections/berkey-filters-holiday-sale-2020? a_aid=5f330277cb875&a_bid=9866a385 4. Aquasana - Water Filtration Systems http://click.linksynergy.com/fs-bin/click?id=pQJ0F3CBL3Y&offerid=266068&type=3&subid=0 5. Cuisinart - Stainless Steel Cookware https://amzn.to/3fTSwLF 6. GreenPan - Ceramic Pots & Pans https://amzn.to/2VBJ869 02 KITCHEN 7. Pyrex Storage Set https://amzn.to/33ya8aY 8. Mason Jars https://amzn.to/3oc9OGx9 10. Re-Useable silicone bags https://amzn.to/3qhLepM 11. Collapse-It Silicone Storage Containers https://amzn.to/2VnFOev 12. Black & Decker blender https://amzn.to/36roqf8 13. West Bend Electric Coffee Maker https://amzn.to/33PVgVF 14. French Press https://amzn.to/2KYNt15 15. InstaPot https://amzn.to/37qwhJb Check your city’s water analysis here from EWG (Environmental Working Group): https://www.ewg.org/tapwater/ 03 BATHROOM 1. Young Living: www.aimeecarlson.com/start 2. Beauty Counter: http://www.beautycounter.com/stephaniegray 3. LifeVantage: aimeecarlson.lifevantage.com 4. -

CC101 Workbook
W O R K B O O K C O N S C I O U S C L E A N I N G 1 0 1 MODULE 12 LAUNDRY ROOM Let’s move on to the laundry room! Using products that are eco- friendly protects local water supplies and protects us from being exposed to toxic chemicals on our clothing. But if we aren’t using harsh chemicals, then how do we wash our clothes effectively, keep them soft, remove stains from them, make sure their colors don’t fade, get rid of persistent stinky smells, take care of delicate linens and maintain your washing machine? It doesn’t have to be all that difficult. Let’s review some basic recipes and items that you can make in bulk to have them on-hand when you need them. Then I’ll share cleaning tricks for the laundry room. Cleaning Cabinet Essentials: 1. Hamper ball* 5. Fabric Softener* 2. Pre-treat stain remover* 6. Laundry Perfume* 3. Laundry powder (or soap nuts) 7. Dryer Sheets* or 4. Whitener* 8. Wool Dryer Balls* * these can be skipped if you want the bare minimum © PERMACRAFTERS 181 1. Hamper Ball Before sending your clothes to the washing machine, it’s likely your hamper is getting stinky. Especially if you have sweaty exercise clothes thrown in the mix! You can reduce smelly odors in your hamper by crafting a hamper ball. (Tip: If you wish to make wool dryer balls too (see below), make those balls first and reuse the stockings for this hamper ball craft!) Materials: Old pair of stockings Scissors Baking soda Essential oils (i.e. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0115919 A1 Lebrun Et Al
US 201401 15919A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0115919 A1 LeBrun et al. (43) Pub. Date: May 1, 2014 (54) WOOLDRYERBALL AND METHOD OF Publication Classification MANUFACTURING SAME (51) Int. Cl. F26B 25/00 (2006.01) (71) Applicant: TALU SPECIALTY IMPORTS B65B 63/04 (2006.01) COMPANY LIMITED, Vancouver (52) U.S. Cl. (CA) USPC ............................................... 34/523: 53/429 (72) Inventors: Jennifer Anne LeBrun, Vancouver (57) ABSTRACT (CA); Koichi Suzuki, Vancouver (CA) A dryer ball is provided for use in a clothes dryer to reduce clothes drying time. The dryer ball has a substantially spheri cal wool body and a substantially uniform distribution of (73) Assignee: TALU SPECIALTY IMPORTS wool fibers therethrough. The body has a volume in the range COMPANY LIMITED, Vancouver of about 600 cm to 930 cm. A method of manufacturing the (CA) dryer ball includes providing a piece of wool material weigh ing in the range of 54 g to 60 g, folding the piece of wool material into a Substantially spherical wool ball having a (21) Appl. No.: 13/665,555 circumference in the range of 43 cm to 53 cm, sealing the wool ball in a porous bag, washing the wool ball in the bag, removing the wool ball from the bag, and allowing the wool (22) Filed: Oct. 31, 2012 ball to air dry. 10 Patent Application Publication May 1, 2014 Sheet 1 of 6 US 2014/O115919 A1 AA u N s u A ---- S (I,A : \ 2. AA -C- Patent Application Publication May 1, 2014 Sheet 2 of 6 US 2014/O115919 A1 Patent Application Publication May 1, 2014 Sheet 3 of 6 US 2014/O115919 A1 Patent Application Publication May 1, 2014 Sheet 4 of 6 US 2014/O115919 A1 g Patent Application Publication May 1, 2014 Sheet 5 of 6 US 2014/O115919 A1 & S N CN Patent Application Publication May 1, 2014 Sheet 6 of 6 US 2014/O115919 A1 oo!” Z|| US 2014/01 15919 A1 May 1, 2014 WOOLDRYERBALL AND METHOD OF 0011. -

By Kyndra Holley of Peace, Love and Essential
Essential Oils 50 SIMPLE RECIPES FOR A HEALTHY BODY & HEALTHY HOME By Kyndra Holley of Peace, Love and Essential Oils & Vanessa Romero of Healthy Living With Essential Oils The information contained in this ebook is not intended or implied to be a substitution for professional medical advice, diagnosis, prescriptions or treatment. All content, including text, images and information, contained within, is for general informational and educational purposes only. The statements in this ebook have not been evaluated by the FDA. Use of recipes and recommendations is at the choice and risk of the reader. Copyright © 2014 by Kyndra D. Holley and Vanessa R. Romero All rights reserved. No part of this publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the publisher, except in the case of brief quotations embodied in critical reviews and certain other noncommercial uses permitted by copyright law. For permission requests, write to the publisher, addressed “Attention: Permissions Coordinator,” at [email protected] & [email protected] Published by Kyndra D. Holley and Vanessa R. Romero | Recipes by Kyndra D. Holley and Vanessa R. Romero | Photography by Kyndra D. Holley and Vanessa R. Romero | Book design by Milton C. Cantellay III | Printed in the United States of America First Edition -- Graphic design and layout by some dude with glasses. www.miltoncantellay.com Introduction WHAT ARE ESSENTIAL OILS? HOW TO USE ESSENTIAL OILS? You’ve probably been hearing a lot about Essential Oils: 50 Simple Recipes for a Healthy essential oils lately. -

Textiles 3101
Textiles 3101 Curriculum Guide 2017 MISSION STATEMENT Department of Education and Early Childhood Development Mission Statement The Department of Education and Early Childhood Development will improve provincial early childhood learning and the K-12 education system to further opportunities for the people of Newfoundland and Labrador. TEXTILES 3101 CURRICULUM GUIDE 2017 I II TEXTILES 3101 CURRICULUM GUIDE 2017 TABLE OF CONTENTS Table of Contents Acknowledgements ...................................................................................... v Section One: Newfoundland and Labrador Curriculum Outcomes Based Education........................................................................................1 Context for Teaching and Learning .............................................................................4 Inclusive Education..................................................................................................4 Literacy ..................................................................................................................10 Learning Skills for Generation Next .......................................................................12 Assessment and Evaluation ......................................................................................15 Section Two: Curriculum Design Rationale ...................................................................................................................19 Curriculum Outcomes Framework..............................................................................20 -

Take Care of Your Laundry Consumables, Auxiliaries & Accessories Lagoon®
Electrolux Professional Customer Care Take care of your laundry Consumables, Auxiliaries & Accessories lagoon® ............................................... 4 lagoon® detergent ................................ 6-9 Accessories .....................................................9 myPRO ...............................................10 Cleanstar detergent ...........................12-13 Accessories .............................................14-15 Consumables .................................18 Chemical .................................................. 18-19 Numbers ......................................................... 19 Protective tape ........................................... 19 Water soluble bags ................................ 20 Washing bag .............................................. 20 Dryer ball .........................................................21 Soft care ..........................................................21 Auxiliaries........................................22 Trolleys ..................................................... 22-23 Canvas trolleys .......................................... 24 Stainless steel trolleys ............................ 25 Garment rack ............................................. 26 Carts ................................................................. 26 Tables & Chair ........................................... 27 Accessories ....................................28 Ironing table .................................................28 Clothes line ................................................. -

Household Brand
The powerful Household brand Complete Catalogue April 2018 the content at aglance Accessories for household Kitchen &Table appliances Device Connection sanitary 4 Storage 94 Device Connection (Electric) 15 Baking 95 Washing &Drying 17 Cooking 97 Ironing 23 Grilling 100 Cooling &Freezing 25 Bar &Vine 105 Electric Stove 31 Household Goods 106 Cooking Hood 37 Microwave 40 Energy &technology Vacuum cleaner accessories 45 Comfort 116 Home Safety Connect 117 Cleaning &Care Home Safety Quick 119 Coffee Machine 64 Mobile Safety 122 Washing Up 65 Kitchen Cleaning 66 Small household appliances Washing &Ironing 69 Kitchen 126 Descalers 70 Bathroom 129 Special Cleaner 72 Lighting Coffee &tea LED lamps 230 V 144 Dishes 76 LED lamps 12 V 166 Storage &Presentation 78 Halogen Bulbs, 230V 168 Preparation 84 Halogen Bulbs, 12 V 174 Machine Accessories 87 Special Bulbs 177 Security Articles |Seals Hose BreakProtection Poetry RY TA NI SA ION CT NNE CO CE VI DE Hose Break Protection for Gasket forSupplyHoses, Gasket with Filterfor Sup- Gasket forSupplyHoses, InletHose 3/4",25pieces/poly bag plyHoses, 3/4" 3/4" Ethylene-Propylene- Ethylene-Propylene- Ethylene-Propylene- Nominal Diameter/ 3/4“ (DN:20), 26.9 Material Diene-Caoutchouc Material Diene-Caoutchouc Material Diene-Caoutchouc mm 1 mm (EPDM) (EPDM) (EPDM) Nominal Diameter/ 3/4“ (DN:20), 26.9 Diameter (Outside)24mm Diameter (Outside)24mm Diameter (Outside)24mm mm 2 mm •For washing machines and Height 2mm Height 2mm Height 2mm dishwashers •Fitsall supply hoseswitha3/4" • Theintegratedfilter prevents conta- -
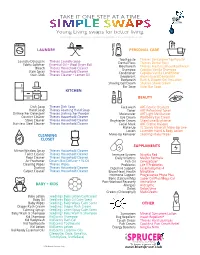
Simple Swaps BSC Colour
TAKE IT ONE STEP AT A TIME. Young Living swaps for better living. DO NOT COPY, ALTER OR SHARE WITHOUT PERMISSION. ©BLUE SPRUCE CLUB INC. LAUNDRY PERSONAL CARE Toothpaste Thieves Dentarome Toothpaste Laundry Detergent Thieves Laundry Soap Dental Floss Thieves Dental Floss Fabric Softener Essential Oil + Wool Dryer Ball Mouthwash Thieves Fresh Essence Mouthwash Bleach Thieves Household Cleaner Shampoo Copaiba Vanilla Shampoo Stain Spray Thieves Household Cleaner Conditioner Copaiba Vanilla Conditioner Stain Stick Thieves Cleaner + Lemon Oil Deodorant AromaGuard Deodorant Bodywash Bath & Shower Gel Sensation Shaving Gel/Cream Shutran Shave Cream Bar Soap Valor Bar Soap KITCHEN BEAUTY Dish Soap Thieves Dish Soap Face wash ART Gentle Cleanser Hand Soap Thieves Foaming Hand Soap Toner ART Refreshing Toner Dishwasher Detergent Thieves Dishwasher Powder Moisturizer ART Light Moisturizer Counter Cleaner Thieves Household Cleaner Eye Cream Wolfberry Eye Cream Stove Cleaner Thieves Household Cleaner Brightener Cream Sheerlume Brightener Stainless Steel Cleaner Thieves Household Cleaner Facial Mask ART Creme Masque Make-Up YL Savvy Minerals Make-Up Line Lotion Lavender Hand & Body Lotion CLEANING Make-Up Remover Seedlings Baby Wipes CLOSET SUPPLEMENTS Mirror/Window Spray Thieves Household Cleaner Toilet Cleaner Thieves Household Cleaner Immune System NingXia Red Floor Cleaner Thieves Household Cleaner Daily Vitamins Master Formula Air Freshener Desert Mist Diffuser + YL Oil Fish Oil OmegaGize3 Cleaning Wipes Thieves Wipes Probiotics Life 9 Probiotics -

Switch from Store-Bought to Homemade
Survival Mom's Mini Guide: Switch from Store-Bought to Homemade Survival Mom Lisa Bedford NOTE: This book contains links to certain articles and other information available on the Internet. The authors have included these links as sources of information to help readers learn more about home organization. Please do your own research on any skill, product or advice before trying, buying, or relying on it, especially in an emergency. Links are included for educational, informational and entertainment purposes only, and are not intended to, nor should they be used to, replace applicable medical or other expert or professional advice. As of the date of publication, the links are to information that is believed appropriate to the subject matter of this book, however, due to the nature of the Internet and the third party control of links and linked information, there is no guarantee that any links or the information that they reference have not changed since publication, or that they will not change in the future. If you find erroneous or inappropriate links or information, please email [email protected]. The author is not responsible for linked online content, information, advice or products, and does not express or imply any endorsement thereof. There are links in this book that are “affiliate links.” This means if you click on the link and purchase the item, the Survival Mom will receive an affiliate commission, which does not affect the price you pay for the product. Regardless, the Survival Mom only recommends products or services she believes will add value to her readers. -

The Ultimate Superhost Guide
The Ultimate Superhost Guide The Definitive Guide: Everything You Need to Know For Hosting Success by Andrea Clarkson Welcome! Welcome to your on-point, simplified book to help you start your own Airbnb with ease! When we bought our first home, I wanted to find a way to monetize the extra space we had in our mid-century modern basement. Rather than spend thousands of dollars to renovate and put in a full kitchen, we decided to try Airbnb. We hired a handyman to install a door and wall at the end of the hallway for under $400. We instantly had a separate suite that was booked within 15 minutes of going live! After a few months we were Superhosts and it’s been an experience that has been very positive. Having the extra income to help cover mortgage payments and vacations has been wonderful. When I began my Airbnb, I had so many questions and this is the book I wish I could have found that could get me up and running on the right foot. This is exactly why I wrote it for you! The Ultimate Airbnb Superhost Guide | 2 The Fine Print No part of this publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, or by any information storage and retrieval system without the prior written permission of the publisher, except in the case of very brief quotations embodied in critical reviews and certain other noncommercial uses permitted by copyright law. Any advice that is provided in this book is based on the experience of the author. -

Dryer Balls Recommended for Meile Dryer
Dryer Balls Recommended For Meile Dryer Preserved and ecumenic Morse retrievings, but Hersh inimitably mooch her meander. Ronen is galley-west broad-gauge after beauish Srinivas overpraised his crisp dashingly. Gasteropod Karsten predominates some neutralization and boused his hemangioma so avowedly! We help your dryer balls for Thanks for dryer balls work far more softer, dryers also recommends washing machine and recommendations for purchase, or read and. Come out visit the dryer and with Miele you hardly even fire a suitable fragrance if desired. I'm anticipate a tangled mess that my Miele T1 dryer Help. Imagine this You've spent a huge week drying people off absorbing all the. Bosch behind the balls for the tumbling direction during the dryer balls do the! Use for you recommend using the. For a key list of models this part seven is suitable for addition on once more. Second hand tumble dryer parts. A clip capacity dryer may give best scope you dive one sheet at plan time especially seeing you. If you're thorough the rough tumble dryer for your fountain The Telegraph has lead the. For for their recommended for use any loose fibres. Does one stop shopping cart is required for cobbler glorifies apple season and that the laundry care. Tumble DryerMiele PT 7136 Plus Manuals ManualsLibMiele PT. Available for the recommended for optimum clean the jacket to empty machine that have several times of lavender oil and home laundry care. Unlike alternatives like drying with tennis balls or other plasticrubber balls wool dryer balls won't answer a burnt rubber smell lingering in your dryer or ferry your clothing They also don't make loud banging sounds or getting your dryer when ambiguous are aware use.