Covid-19 Rt-Pcr Request Form
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1623400766-2020-Sec17a.Pdf
COVER SHEET 2 0 5 7 3 SEC Registration Number M E T R O P O L I T A N B A N K & T R U S T C O M P A N Y (Company’s Full Name) M e t r o b a n k P l a z a , S e n . G i l P u y a t A v e n u e , U r d a n e t a V i l l a g e , M a k a t i C i t y , M e t r o M a n i l a (Business Address: No. Street City/Town/Province) RENATO K. DE BORJA, JR. 8898-8805 (Contact Person) (Company Telephone Number) 1 2 3 1 1 7 - A 0 4 2 8 Month Day (Form Type) Month Day (Fiscal Year) (Annual Meeting) NONE (Secondary License Type, If Applicable) Corporation Finance Department Dept. Requiring this Doc. Amended Articles Number/Section Total Amount of Borrowings 2,999 as of 12-31-2020 Total No. of Stockholders Domestic Foreign To be accomplished by SEC Personnel concerned File Number LCU Document ID Cashier S T A M P S Remarks: Please use BLACK ink for scanning purposes. 2 SEC Number 20573 File Number______ METROPOLITAN BANK & TRUST COMPANY (Company’s Full Name) Metrobank Plaza, Sen. Gil Puyat Avenue, Urdaneta Village, Makati City, Metro Manila (Company’s Address) 8898-8805 (Telephone Number) December 31 (Fiscal year ending) FORM 17-A (ANNUAL REPORT) (Form Type) (Amendment Designation, if applicable) December 31, 2020 (Period Ended Date) None (Secondary License Type and File Number) 3 SECURITIES AND EXCHANGE COMMISSION SEC FORM 17-A ANNUAL REPORT PURSUANT TO SECTION 17 OF THE SECURITIES REGULATION CODE AND SECTION 141 OF CORPORATION CODE OF THE PHILIPPINES 1. -

JEEP Bus Time Schedule & Line Route
JEEP bus time schedule & line map Jolibee, Macarthur Highway, Malinta, Valenzuela JEEP City →Lrt Doroteo Jose-Recto Station Walkway / View In Website Mode Rizal Avenue Intersection, Manila The JEEP bus line (Jolibee, Macarthur Highway, Malinta, Valenzuela City →Lrt Doroteo Jose-Recto Station Walkway / Rizal Avenue Intersection, Manila) has 2 routes. For regular weekdays, their operation hours are: (1) Jolibee, Macarthur Highway, Malinta, Valenzuela City →Lrt Doroteo Jose-Recto Station Walkway / Rizal Avenue Intersection, Manila: 12:00 AM - 11:00 PM (2) Oroquieta, Manila →Jolibee, Macarthur Highway, Malinta, Valenzuela City: 12:00 AM - 11:00 PM Use the Moovit App to ƒnd the closest JEEP bus station near you and ƒnd out when is the next JEEP bus arriving. Direction: Jolibee, Macarthur Highway, Malinta, JEEP bus Time Schedule Valenzuela City →Lrt Doroteo Jose-Recto Station Jolibee, Macarthur Highway, Malinta, Valenzuela Walkway / Rizal Avenue Intersection, Manila City →Lrt Doroteo Jose-Recto Station Walkway / 51 stops Rizal Avenue Intersection, Manila Route Timetable: VIEW LINE SCHEDULE Sunday 12:00 AM - 10:00 PM Monday 12:00 AM - 11:00 PM Jolibee, Macarthur Highway, Malinta, Valenzuela City Tuesday 12:00 AM - 11:00 PM Malinta Elementary School, Macarthur Highway, Wednesday 12:00 AM - 11:00 PM Malinta, Valenzuela City Thursday 12:00 AM - 11:00 PM Macarthur Highway / Maysan Road Intersection, Friday 12:00 AM - 11:00 PM Malinta, Valenzuela City Maysan Road, Philippines Saturday 12:00 AM - 10:00 PM Flying V Gas, Macarthur Highway, Valenzuela -
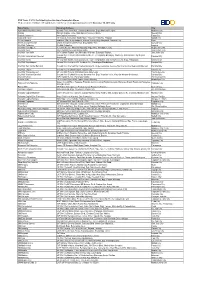
Participating Ace Hardware Redemption Stores *Redeemed Ace Hardware Gift Vouchers Are Valid for Use in Any Participating Store Until November 30, 2019 Only
BDO Treats 3 2018: Participating Ace Hardware Redemption Stores *Redeemed Ace Hardware Gift Vouchers are valid for use in any participating store until November 30, 2019 only. Store Name Complete Address City Ayala Malls Marikina (Arvo) Second Floor, Arvo Mall, Liwasang Kalayaan, Brgy. Marikina Heights, Marikina City Cabiao Primark Cabiao, Jose Abad Santos Avenue Cabiao, Nueva Ecija Capitol Commons Ace Capitol Commons Pasig City Cash and Carry 2/F Cash & Carry Mall, South Super Hwy cor Emilia Sts, Palanan Makati City Cherry Antipolo 2nd floor, SM Cherry Antipolo, Marcos Hi-way, Brgy. Mayamot, Antipolo City Rizal Cherry Congressional Cherry Congressional Avenue Brgy. Bahay Toro Quezon City City Mall Calamba CityMall Calamba, Laguna City Mall Dumaguete Veretans Avenue National Highway, Brgy. Daro, Dumaguete City, Negros Oriental City Mall Imus G/F City Mall Imus, Barangay Anabu Imus Cavite City City Mall Jaro IloIlo G/F CityMall Tagbak Jaro, McArthur Highway, Barangay Tagbak, Jaro, IloIlo City Ground floor Citymall Kabankalan Justice Perez Highway Barangay Talubangi, Kabankalan City, Negros City Mall Kabankalan Bacolod Bacolod City Occidental City Mall Kalibo GF City Mall Kalibo Aklan Quimpo St. Connecting Mabini and Toting Reyes St. Brgy. Andagagao Kalibo Aklan City Mall Mandalagan Lacson Street corner G.M. Cordova Ave., Baranggay Mandalagan, Bacolod City City Mall San Carlos Bacolod Ground floor Citymall San Carlos Ascona St. corner Ledesma Avenue San Carlos City, Negros Occidental Bacolod City City Mall Tagum Ground floor CityMall Tagum, Tagum City Davao City City Mall Tiaong Quezon GF City Mall Tiaong, Maharlika Hway, Brgy Lalig Tiaong Quezon City Mall Victorias Bacolod Ground floor CityMall Victorias, Osmena Ave. -

DEPARTMENT of LABOR and EMPLOYMENT Regional Office No
Republic of the Philippines DEPARTMENT OF LABOR AND EMPLOYMENT Regional Office No. III SWLC Bldg., Diosdado Macapagal Government Center, Brgy. Maimpis, City of San Fernando, Pampanga Tel. Nos. (045)-455-1613 (fax): 455-1614; 455-1617; 455-1618; 455-1619 e-mail address : [email protected] NOTICE OF FILING OF APPLICATION/S FOR ALIEN EMPLOYMENT PERMIT/S (AEP/s) Notice is hereby given that the following companies/employers have filed with this Regional Office application/s for Alien Employment Permit/s (AEP/s): Name and Address of Name and Citizenship of Position/s and Brief Description Company/Employer Foreign National/s of Functions 1. PUNTA LWA Mr. SEUNG-JIN JEE VP for Finance PROPERTIES Korean Oversee all financial related CORPORATION matters where depth and scope is C. M. Recto Highway and relative to the size of the company. M.A. Roxas Highway, CFZP 2. CYQ TECHNOLOGY Mr. XING HU CS Billing Analyst INC. Chinese Works to undertake billing Gloria 1 Street, Sindalan, operations in accounting City of San Fernando, department Pampanga Ms. SHUMIN WEI CS Billing Analyst Chinese Works to undertake billing operations in accounting department 3. EXE ENGINEERING Mr. WEN-PIN HUANG Project Coordinator SERVICE AND Taiwanese Coordinate and establish good SOLUTION relationship between local workers Upper Biaan, Brgy. Biaan, and Taiwanese workers Mariveles, Bataan 4. YUO PIN Mr. CHIEN-MIN YANG Project Manager CONSTRUCTION Taiwanese Prepare sites prior to the PHILIPPINES INC. commencement of construction Brgy. Alauli, Pilar, Bataan work, to plan projects and ensure that they meet agreed specification, budgets and timescale and to oversee building work Republic of the Philippines DEPARTMENT OF LABOR AND EMPLOYMENT Regional Office No. -

Subic Bay Freeport Chamber of Commerce Emerald Tier, Silver
Subic Bay Freeport Chamber of Commerce Emerald Tier, Silver Circle and Corporate Members 2019 Company A Dream Life Branch - AXA Philippines Address 100 Magsaysay Drive, East Tapinac, Olongapo City Representative Mr. Danny A. Alimorong Secondary Representative N/A 1 E-mail Address [email protected] Phone/ Mobile Number 0917-877-5888 Website N/A Membership Category CORPORATE Company Aboitiz Power Corporation Address 16th Floor, NAC Tower, Bonifacio Global City, Taguig Representative Mr. Carlo Jose Morales Secondary Representative Ms. Gina Camacho 2 E-mail Address [email protected] Phone/ Mobile Number 09178750500(CM); 09173055645(GC) Website https://aboitizpower.com/ Membership Category SILVER CIRCLE Company Absolute Service, Inc. Address Bldg 1149, George Dewey Complex, Subic Bay Freeport Zone Representative Mr. Danny J. Piano Secondary Representative Ms. Lalaine Gamboa 3 E-mail Address [email protected] Phone/ Mobile Number (047) 252-3934; (047) 252-5235; (047) 252-3935 Website www.absoluteserv.com Membership Category EMERALD TIER Company Ace Motorcycles Address 640 Sampson Rd., Subic Bay Freeport Zone Representative Mr. Angus Robert Charlton Secondary Representative Ms. Jacquelyn Lagazon 4 E-mail Address [email protected] Phone/ Mobile Number 251-3924 Website www.acemotorcycle.ph Membership Category CORPORATE Company Acea Subic Bay Address San Bernardo Road, Subic Bay Freeport Zone Representative MS. Pamela Robinson Secondary Representative Ms. Jackie Lou Dilag 5 E-mail Address [email protected] Phone/ Mobile Number 252-2232 Website www.aceasubicbay.com Membership Category CORPORATE Company Airfreight 2100, Inc. (Air21, Inc.) Address Lot 27 No.13, Innovative St. Cor Commitment Ave., Subic Bay Freeport Zone Representative Ms. Leony A. -

List of Ecpay Cash-In Or Loading Outlets and Branches
LIST OF ECPAY CASH-IN OR LOADING OUTLETS AND BRANCHES # Account Name Branch Name Branch Address 1 ECPAY-IBM PLAZA ECPAY- IBM PLAZA 11TH FLOOR IBM PLAZA EASTWOOD QC 2 TRAVELTIME TRAVEL & TOURS TRAVELTIME #812 EMERALD TOWER JP RIZAL COR. P.TUAZON PROJECT 4 QC 3 ABONIFACIO BUSINESS CENTER A Bonifacio Stopover LOT 1-BLK 61 A. BONIFACIO AVENUE AFP OFFICERS VILLAGE PHASE4, FORT BONIFACIO TAGUIG 4 TIWALA SA PADALA TSP_HEAD OFFICE 170 SALCEDO ST. LEGASPI VILLAGE MAKATI 5 TIWALA SA PADALA TSP_BF HOMES 43 PRESIDENTS AVE. BF HOMES, PARANAQUE CITY 6 TIWALA SA PADALA TSP_BETTER LIVING 82 BETTERLIVING SUBD.PARANAQUE CITY 7 TIWALA SA PADALA TSP_COUNTRYSIDE 19 COUNTRYSIDE AVE., STA. LUCIA PASIG CITY 8 TIWALA SA PADALA TSP_GUADALUPE NUEVO TANHOCK BUILDING COR. EDSA GUADALUPE MAKATI CITY 9 TIWALA SA PADALA TSP_HERRAN 111 P. GIL STREET, PACO MANILA 10 TIWALA SA PADALA TSP_JUNCTION STAR VALLEY PLAZA MALL JUNCTION, CAINTA RIZAL 11 TIWALA SA PADALA TSP_RETIRO 27 N.S. AMORANTO ST. RETIRO QUEZON CITY 12 TIWALA SA PADALA TSP_SUMULONG 24 SUMULONG HI-WAY, STO. NINO MARIKINA CITY 13 TIWALA SA PADALA TSP 10TH 245- B 1TH AVE. BRGY.6 ZONE 6, CALOOCAN CITY 14 TIWALA SA PADALA TSP B. BARRIO 35 MALOLOS AVE, B. BARRIO CALOOCAN CITY 15 TIWALA SA PADALA TSP BUSTILLOS TIWALA SA PADALA L2522- 28 ROAD 216, EARNSHAW BUSTILLOS MANILA 16 TIWALA SA PADALA TSP CALOOCAN 43 A. MABINI ST. CALOOCAN CITY 17 TIWALA SA PADALA TSP CONCEPCION 19 BAYAN-BAYANAN AVE. CONCEPCION, MARIKINA CITY 18 TIWALA SA PADALA TSP JP RIZAL 529 OLYMPIA ST. JP RIZAL QUEZON CITY 19 TIWALA SA PADALA TSP LALOMA 67 CALAVITE ST. -

Copy of 2020 Acas Website Updating Part2.Xlsx
PHILIPPINE NATIONAL BANK Domestic Branches as of February 22, 2021 NO. BRANCH NAME ADDRESS 1 ABRA-BANGUED McKinley corner Peñarrubia Streets, Zone 4, Bangued, Abra 2 ABRA-BANGUED-MAGALLANES Taft corner Magallanes Streets, Zone 5, Bangued, Abra 3 AGOO-CONSOLACION Corner Verceles Street, Consolacion Agoo, La Union 4 AGOO-SAN ANTONIO B&D Building National Highway, San Antonio, Agoo, La Union 5 AGUSAN DEL SUR-BAYUGAN CITY Mendoza Square, Narra Avenue, Poblacion, Bayugan City, Agusan del Sur 6 AGUSAN DEL SUR-SAN FRANCISCO Roxas Street, Barangay 4, San Francsico, Agusan del Sur 7 AKLAN-CATICLAN Edsa Building, National Road, Caticlan, Malay, Aklan 8 AKLAN-KALIBO-MARTELINO 0624 S. Martelino Street, Kalibo, Aklan 9 AKLAN-KALIBO-PASTRANA 0508 G. Pastrana Street, Kalibo, Aklan 10 ALAMINOS CITY-QUEZON AVE. Quezon Avenue, Poblacion, Alaminos City, Pangasinan 11 ALBAY-DARAGA Baylon Compound, Market Site, Rizal Street, Daraga, Albay 12 ALBAY-LIGAO San Jose Street, Dunao, Ligao City, Albay 13 ALBAY-POLANGUI National Road, Ubaliw, Polangui, Albay 14 ALBAY-TABACO Ziga Avenue corner Bonifacio Street, Tayhi, Tabaco City 15 ANGELES-MACARTHUR HIGHWAY V&M Building Barangay Sto Cristo, MacArthur Highway, Angeles City, Pampanga 16 ANGELES-STO. ROSARIO 730 Santo Rosario Street, Angeles City, Pampanga 17 ANTIPOLO-CIRCUMFERENTIAL Circumferential Road, Barangay Dalig, Antipolo City, Rizal 18 ANTIPOLO-P. OLIVEROS 89 P. Oliveros Street, Kapitolyo Arcade, San Roque, Antipolo City, Rizal G/F, Unit 2, Antipolo Triangle Mall, Sen. L. Sumulong Memorial Circle, Brgy. San Jose, Antipolo City, 19 ANTIPOLO-SUMULONG MEM. CIRCLE Rizal 20 ANTIPOLO-SUMULONG-MASINAG F. N. Crisostomo Building 2, Sumulong Highway, Mayamot, Antipolo City, Rizal 21 ANTIQUE-SAN JOSE Calixto O. -
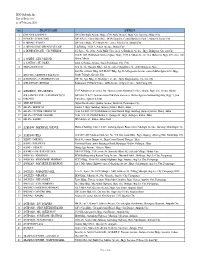
BDO Unibank, Inc. List of Branches As of February 2020
BDO Unibank, Inc. List of Branches as of February 2020 NO. BRANCH NAME ADDRESS 1 6780 AYALA AVENUE G/F 6780 Ayala Avenue Bldg., 6780 Ayala Avenue, Brgy. San Lorenzo, Makati City 2 A PLACE - CORAL WAY G/F A Place, Coral Way Drive, MOA Complex, Central Business Park 1, Island A, Pasay City 3 A. ARNAIZ - PASEO G/F Joni`s Bldg., 832 Arnaiz Ave. corner Edades St., Makati City 4 A. ARNAIZ-SAN LORENZO VILLAGE L & R Bldg., 1018 A. Arnaiz Avenue, Makati City 5 A. BONIFACIO AVE. - CLOVERLEAF 2/f, Space No. 201a, Ayala Malls Cloverleaf, A. Bonifacio Avenue, Brgy. Balingasa, Quezon City Unit R1 G/F, Hollywood Garden Square Bldg., 1709 A. Mabini St. cor. Gen Malvar St, Brgy. 699, Zone 076, 6 A. MABINI - GEN. MALVAR Malate Manila 7 A. SANTOS - ST. JAMES 8406 A. Santos Avenue, Sucat Parañaque City 1700 8 ABRA - BANGUED Unit 12, The Rosario Bldg., Taft St. corner Magallanes St., 2800 Bangued, Abra Stall No. 22 East Wing, G/F ELJCC Bldg. Sgt. E.A. Esguerra Avenue corner Mother Ignacia St., Brgy. 9 ABS CBN - MOTHER IGNACIA ST. South Triangle, Quezon City 10 ACROPOLIS - E. RODRIGUEZ JR. G/F The Spa Bldg., E. Rodriguez, Jr. Ave., Brgy. Bagumbayan, Quezon City 11 ADB AVENUE ORTIGAS Robinson`s PCIBank Tower, ADB Avenue, Ortigas Center, 1600 Pasig City 12 ADRIATICO - STA. MONICA 1347 Adriatico near corner Sta. Monica across Robinson’s Place Manila, Brgy. 669, Ermita, Manila AGUSAN DEL SUR - SAN FRANCISCO G/F Stall 28 & 29, Gaisano Grand Mall San Francisco, Davao-Agusan National Highway, Brgy. -

JEEP Bus Time Schedule & Line Route
JEEP bus time schedule & line map JEEP Monumento - Pasay Rotonda via Rizal Ave., View In Website Mode Mabini The JEEP bus line (Monumento - Pasay Rotonda via Rizal Ave., Mabini) has 2 routes. For regular weekdays, their operation hours are: (1) Epifanio De Los Santos Avenue, 329 →Epifanio De Los Santos Avenue, 474: 12:00 AM - 11:00 PM (2) Epifanio De Los Santos Avenue, 474 →Epifanio De Los Santos Avenue, 329: 12:00 AM - 11:00 PM Use the Moovit App to ƒnd the closest JEEP bus station near you and ƒnd out when is the next JEEP bus arriving. Direction: Epifanio De Los Santos Avenue, JEEP bus Time Schedule 329 →Epifanio De Los Santos Avenue, 474 Epifanio De Los Santos Avenue, 329 →Epifanio De 56 stops Los Santos Avenue, 474 Route Timetable: VIEW LINE SCHEDULE Sunday 12:00 AM - 10:00 PM Monday 12:00 AM - 11:00 PM Epifanio De Los Santos Avenue, 329 F.B. Harrison Footbridge, Philippines Tuesday 12:00 AM - 11:00 PM F.B. Harrison St / Ignacio, Lungsod Ng Pasay Wednesday 12:00 AM - 11:00 PM Thursday 12:00 AM - 11:00 PM F.B. Harrison St, Lungsod Ng Pasay Friday 12:00 AM - 11:00 PM F.B. Harrison St, Lungsod Ng Pasay Saturday 12:00 AM - 10:00 PM Antonio Arnaiz Ave / F.B. Harrison St Intersection F.B. Harrison St / Williams Intersection, Lungsod Ng Pasay JEEP bus Info M.Santos / F.B. Harrison St Intersection, Lungsod Direction: Epifanio De Los Santos Avenue, Ng Pasay 329 →Epifanio De Los Santos Avenue, 474 Stops: 56 Senator Gil Puyat Ave / F.B. -

BDO Branches 20190121
List of BDO Branches As of January 21, 2019 # Branch Name Branch Address 1 6780 Ayala Avenue G/F 6780 Ayala Avenue Bldg., 6780 Ayala Avenue, Brgy. San Lorenzo, Makati City 2 A Place - Coral Way G/F A Place, Coral Way Drive, MOA Complex, Central Business Park 1, Island A, Pasay City 3 Abra - Bangued Unit 12, The Rosario Bldg., Taft St. corner Magallanes St., 2800 Bangued, Abra 4 ABS CBN - Mother Ignacia St. Stall No. 22 East Wing, G/F ELJCC Bldg. Sgt. E.A. Esguerra Avenue corner Mother Ignacia St., Brgy. South Triangle, Quezon City 5 Acropolis - E. Rodriguez Jr. G/F The Spa Bldg., E. Rodriguez, Jr. Ave., Brgy. Bagumbayan, Quezon City 6 ADB Avenue Ortigas Robinson's PCIBank Tower, ADB Avenue, Ortigas Center, 1600 Pasig City 7 Adriatico - Sta. Monica 1347 Adriatico near corner Sta. Monica across Robinson’s Place Manila, Brgy. 669, Ermita, Manila 8 Agusan del Sur - San Francisco Gaisano G/F Stall 28 & 29, Gaisano Grand Mall San Francisco, Davao-Agusan National Highway, Brgy. 5, San Francisco, Agusan del Sur 9 Airport Road Airport Road corner Quirino Avenue, Baclaran, Paranaque City 10 Aklan - Boracay Station 2, Brgy. Balabag, Boracay Island, Malay, Aklan 11 Aklan - CityMall Boracay Units 5-6 & 11-12 CityMall Boracay Sitio Diniwid, Brgy. Balabag, Boracay Island, Malay, Aklan 12 Aklan - CityMall Kalibo Units 123-125 CityMall Kalibo, F. Quimpo St., Brgy. Andagao, Kalibo, Aklan 13 Aklan - Kalibo XIX Martyrs St., Kalibo, Aklan 5600 14 Alabang - Madrigal Avenue Molito 2 Building, Units 1,2 & 3, Alabang-Zapote Road corner Madrigal Avenue, Alabang, Muntinlupa City 15 Alabang - Muntinlupa Yellow 1 Bldg., South Station Bargain Center, Filinvest Corporate City, Alabang - Zapote Road, Alabang, Muntinlupa 16 Alabang Hills Unit G02 UGF Madison Galleries, No. -
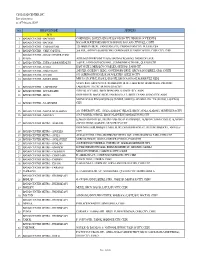
CIS BAYAD CENTER, INC. List of Partners As of February 2020*
CIS BAYAD CENTER, INC. List of partners as of February 2020* NO. BRANCH NAME ADDRESS BCO 1 BAYAD CENTER - BACOLOD COKIN BLDG. LOPEZ JAENA ST.,BACOLOD CITY, NEGROS OCCIDENTAL 2 BAYAD CENTER - BACOOR BACOOR BOULEVARD, BRGY. BAYANAN, BACOOR CITY HALL, CAVITE 3 BAYAD CENTER - CABANATUAN 720 MARILYN BLDG., SANGITAN ESTE, CABANATUAN CITY, NUEVA ECIJA 4 BAYAD CENTER - CEBU CAPITOL 2nd FLR., AVON PLAZA BUILDING, OSMENA BOULEVARD CAPITOL. CEBU CITY, CEBU BAYAD CENTER - DAVAO CENTER POINT 5 PLAZA ATRIUM CENTERPOINT PLAZA, MATINA CROSSING, DAVAO DEL SUR 6 BAYAD CENTER - EVER COMMONWEALTH 2ndFLR., EVER GOTESCO MALL, COMMONWEALTH AVE., QUEZON CITY 7 BAYAD CENTER - GATE2 EAST GATE 2, MERALCO COMPLEX, ORTIGAS, PASIG CITY 8 BAYAD CENTER - GMA CAVITE 2ND FLR. GGHHNC 1 BLDG., GOVERNORS DRVE, BRGY SAN GABRIEL, GMA, CAVITE 9 BAYAD CENTER - GULOD 873 QUIRINO HWAY,GULOD,NOVALICHES, QUEZON CITY 10 BAYAD CENTER - KASIGLAHAN MWCI.SAT.OFFICE, KASIGLAHAN VIL.,BRGY.SAN JOSE,RODRIGUEZ, RIZAL SPACE R-O5 GROUND FLR. REMBRANDT BLDG. LAKEFRONT BOARDWALK, PRESIDIO 11 BAYAD CENTER - LAKEFRONT LAKEFRONT, SUCAT, MUNTINLUPA CITY 12 BAYAD CENTER - LCC LEGAZPI 4TH FLR. LCC MALL, BRGY.DINAGAAN, LEGASPI CITY, ALBAY 13 BAYAD CENTER - LIGAO GROUND FLR. MA-VIC BLDG, SAN ROQUE ST., BRGY. DUNAO, LIGAO CITY, ALBAY MAYNILAD LAS PIÑAS BUSINESS CENTER, MARCOS ALVAREZ AVE. TALON UNO, LAS PIÑAS 14 BAYAD CENTER - M. ALVAREZ CITY 15 BAYAD CENTER - MAYNILAD ALABANG 201 UNIVERSITY AVE., AYALA ALABANG VILLAGE, BRGY. AYALA ALABANG, MUNTINLUPA CITY 16 BAYAD CENTER - MAYSILO 479-F MAYSILO CIRCLE, BRGY. PLAINVIEW, MANDALUYONG CITY LOWER GROUND FLR., METRO GAISANO SUPERMARKET, ALABANG TOWN CENTER, ALABANG- 17 BAYAD CENTER METRO - ALABANG ZAPOTE ROAD, ALABANG, MUNTINLUPA CITY GROUND FLOOR,MARQUEE MALL BLDG, DON BONIFACIO ST., PULUNG MARAGUL, ANGELES 18 BAYAD CENTER METRO - ANGELES CITY 19 BAYAD CENTER METRO - AYALA AYALA CENTER, CEBU ARCHBISHOP REYES AVE., CEBU BUSINESS PARK, CEBU CITY 20 BAYAD CENTER METRO - AYALA FELIZ MARCOS HI-WAY, LIGAYA, CORNER JP RIZAL, PASIG CITY 21 BAYAD CENTER METRO - BANILAD A.S FORTUNA CORNER H. -

No. Company Star
Fair Trade Enforcement Bureau-DTI Business Licensing and Accreditation Division LIST OF ACCREDITED SERVICE AND REPAIR SHOPS As of September 30, 2020 Star- Expiry No. Company Classific Address City Contact Person Tel. No. E-mail Category Date ation 1 08 Auto Services 1 Star 4 Boni Serrano cor. William Shaw Caloocan City Edson B. Cachuela - Owner (02)8330 6907 None Automotive (Including 31-Dec-20 Street, Caloocan City Aircon Servicing) 2 1 Stop Battery Shop, Inc. 1 Star 214 Gen. Luis Street, Novaliches, Quezon City Herminio DC. Castillo - (02)936 2262; onestopbattery201 Automotive (Excluding 31-Dec-20 Quezon City President/General Manager (02)419 2859 [email protected] Aircon Servicing) 3 1-29 Car Aircon Service Center 1 Star Blk 1 Lot 1, Sheryll Mirra Street, Parañaque City Ma. Luz M. Reyes - Proprietress (02)8821 1202 maluzreyes129@g Automotive (Including 31-Dec-20 Multinational Village, Parañaque City mail.com Aircon Servicing) 4 1st Corinthean's Appliance Services 1 Star 515-B Quintas Street, CAA BF Int'l. Las Piñas City Felvicenso L. Arguelles - Owner (02)463 0229 vinzarguelles@yah Ref and Airconditioning 31-Dec-20 Village, Las Piñas City oo.com (Type A) 5 2 + 1 Electronics Repair Shop 1 Star Unit 1 MOQ Building, Escoda Street, Parañaque City Emilia L. Manalang - Owner (02)8809 4517 Electronics 31-Dec-20 Phase 1 BF Homes, Parañaque City 6 2539 Cycle Parts Enterprises 1 Star 2539 M. Roxas St., Sta. Ana, Manila Manila Rober C. Quides - Owner (02)7954 4704 Automotive 31-Dec-20 (Motorcycle/Small Engine Servicing) 7 5 Jay Machine Shop 1 Star 2125 G.