Guidelines for Monitoring Autophagy in Higher Eukaryotes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essays in Public Economics by Dorian Carloni a Dissertation Submitted In
Essays in Public Economics by Dorian Carloni A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Economics in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Alan J. Auerbach, Chair Professor Hilary W. Hoynes Professor Rucker C. Johnson Professor Emmanuel Saez Spring 2016 Essays in Public Economics Copyright 2016 by Dorian Carloni 1 Abstract Essays in Public Economics by Dorian Carloni Doctor of Philosophy in Economics University of California, Berkeley Professor Alan J. Auerbach, Chair This dissertation consists of three chapters and analyzes individuals' and firms’ response to tax and government spending policies. The first chapter focuses on the economic incidence of a large value added tax (VAT) cut in the restaurant industry in France. In particular it estimates the share of the tax cut falling on workers, firm owners and consumers by analyzing the VAT cut applied to French sit-down restaurants { a drop from 19.6 percent to 5.5 percent { in July 2009. Theoretically, we develop a standard partial equilibrium model of consumption tax incidence that includes consumption substitutability between the taxed good and an untaxed good, and markets for production inputs, which we allow to vary with the tax. Empirically, we use firm-level data and a difference-in-differences strategy to show that the reform increased restaurant profits and the cost of employees, and aggregate price data to estimate the decrease in prices produced by the reform. We compare sit-down restaurants to (a) non-restaurant market services and (b) non-restaurant small firms, and find that prices decreased by around 2 percent, the cost per employee increased by 3.9 percent and the return to capital increased by around 10 percent. -

Ocn752505145-2018-11-02-By-Last-Name.Pdf (248.0Kb)
System Inspectors Sorted by Last Name Total Count: 1424 SI# Expires Name Association Town State 14215 5/1/2021 Zachary D. Aaronson CDM Smith Providence RI 4698 6/30/2019 James Abare Winchendon MA 4046 6/30/2019 Stacey J. Abato Raggs, Inc. Concord MA 2548 6/30/2019 Carl Adamo Lincoln RI 349 6/30/2019 Stephen M. Adams Norwell MA 13724 6/30/2019 David R. Adler Charlton MA 14072 12/1/2019 Nicholas Aguiar Yarmouth DPW - Engineering Division West Yarmouth MA 4332 6/30/2019 James D. Aguiar, Jr. Tri-Spec Corp. Westport MA 14016 5/1/2020 Thorsen Akerley Williams & Sparages, LLC Middleton MA 13661 6/30/2019 Patrick Alderman Swansea MA 2040 6/30/2019 Michael Alesse East Coast Excavating Groveland MA 4789 6/30/2019 Lisa C. Allain Millbury MA 4955 6/30/2019 Mark Allain Peabody MA 12864 6/30/2020 William Allen Bill Allen Enterprises LLC Spring Hill FL 13850 6/30/2021 Edward W. Alleva Jr. Alleva Excavating Company Lunenburg MA 350 6/30/2019 Karl J. Alm Alm and Son Septic West Brookfield MA 14169 12/1/2020 Wyatt J. Alm West Brookfield MA 13975 4/1/2019 Elizabeth Alves All Clear Title 5 Services Norton MA 14252 8/1/2021 Nathon Amaral Mystic Excavating Corp Raynham MA 13218 6/30/2021 Erik Anderson Norwell MA 4658 6/30/2019 Michael Andrade Graves Engineering, Inc. Sutton MA 14210 5/1/2021 Samantha Andrews T. Silvia Excavating Swansea MA 2666 6/30/2019 John R. Andrews, III Andrews Survey & Engineers, Inc. -

Entering New Waters at Pier 94 Leslie Weir
the newsletter of the golden gate audubon society // vol. 103 no. 4 fall 2019 EntEring nEw watErs at PiEr 94 leslie weir ore than a million individual shore- M birds rely on the San Francisco Bay for at least some portion of their annual lifecycle. The Bay Area is classified as a site of hemispheric significance for migra- tory shorebirds by the Western Hemisphere Shorebird Reserve Network, and the Ramsar Convention on Wetlands has designated San Francisco Bay as a site of international sig- nificance for migratory waterfowl. More than 1,000 species of birds, mammals, and fish rely on the San Francisco Bay Estuary—the centerpiece of our region. CONTINUED on page 5 American Avocet at Pier 94. Bob Gunderson long enough to be restored for his aston- ishing journey. Ornithologists discovered that Blackpoll Warblers have the longest migratory route of any New World warbler. Every fall, these tiny 12-gram birds make a nonstop transatlantic flight from upper Canada to South America. Then, the fol- lowing spring, they reverse migrate to their breeding grounds. A few hours later, I checked on this exquisite feathered jewel. He was no longer quiet. I could hear him bouncing against the box top in a frenzied urge to leap up and fly south. I let him go and off he went—toward the Statue of Liberty, a fitting testament to his fortitude. I hoped that I had helped him a little. Cindy Margulis Like this Blackpoll Warbler’s drive to Male Blackpoll Warbler. migrate during the fall season, this is our time of transition at GGAS. -

Non-Hebbian Long-Term Potentiation of Inhibitory Synapses in the Thalamus
The Journal of Neuroscience, October 2, 2013 • 33(40):15675–15685 • 15675 Cellular/Molecular Non-Hebbian Long-Term Potentiation of Inhibitory Synapses in the Thalamus Andrea Rahel Sieber,1 Rogier Min,1 and Thomas Nevian1,2 1Department of Physiology and 2Center for Cognition, Learning and Memory, University of Bern, 3012 Bern, Switzerland The thalamus integrates and transmits sensory information to the neocortex. The activity of thalamocortical relay (TC) cells is modulated by specific inhibitory circuits. Although this inhibition plays a crucial role in regulating thalamic activity, little is known about long-term changes in synaptic strength at these inhibitory synapses. Therefore, we studied long-term plasticity of inhibitory inputs to TC cells in the posterior medial nucleus of the thalamus by combining patch-clamp recordings with two-photon fluorescence microscopy in rat brain slices.WefoundthatspecificactivitypatternsinthepostsynapticTCcellinducedinhibitorylong-termpotentiation(iLTP).ThisiLTPwas non-Hebbian because it did not depend on the timing between presynaptic and postsynaptic activity, but it could be induced by postsyn- aptic burst activity alone. iLTP required postsynaptic dendritic Ca 2ϩ influx evoked by low-threshold Ca 2ϩ spikes. In contrast, tonic postsynaptic spiking from a depolarized membrane potential (Ϫ50 mV), which suppressed these low-threshold Ca 2ϩ spikes, induced no plasticity. The postsynaptic dendritic Ca 2ϩ increase triggered the synthesis of nitric oxide that retrogradely activated presynaptic guanylylcyclase,resultinginthepresynapticexpressionofiLTP.ThedependenceofiLTPonthemembranepotentialandthereforeonthe -

Priests and Deacons INDEX Archdiocesan Priests
Priests and Deacons INDEX Archdiocesan Priests ..................................................................................................... 1 Priests of the Archdiocese in the Order of Ordination ................................................. 14 Priests and Deacons: Alphabetical Necrology ............................................................ 21 Priests and Deacons: Chronological Necrology by Month, Day and Year .................. 51 Archdiocesan Permanent Deacons ............................................................................ 79 11/30/2020 Archdiocesan Priests Adams, Rev. John E. (1969) Baer, Rev. Timothy K. (1996) Director, S.O.M.E., DC Pastor, St. Nicholas, Laurel Phone: 202-832-9710 Phone: 301-490-5116 Aguirre, Rev. Francisco E. (2013) Baraki, Rev. Tesfamariam (1975) Pastor, St. Catherine Labouré, Chaplain, Howard University Hospital, Wheaton DC Phone: 301-946-3636 In Residence, St. Gabriel, DC Phone: 202-726-9092 Agustin Rev. Patrick S. (2020) Parochial Vicar (pro-tem), St. Francis of Barbieri de Carvalho, Rev. Rafael Assisi, Dearwood (2013) Phone: 301-840-1407 Director of Spiritual Formation, Redemptoris Mater Archdiocesan Ailer, Rev. Gellert J. (2006) Missionary Seminary, Hyattsville Missionary Assignment, Archdiocese of Eger, Hungary Barry, Rev. John M. (1988) Pastor, Church of the Resurrection, Alliata, Rev. Peter R. (1961) Burtonsville Retired, St. John Vianney, Prince Phone: 301-236-5200 Frederick Phone: 301-440-1784 Bava, Rev. David A. (1973) Pastor, Holy Redeemer, DC Amey, Rev. Msgr. Robert -
Obituaries Buffalo News 2010 by Name
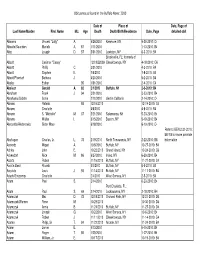
Obituaries as found in the Buffalo News: 2010 Date of Place of Date, Page of Last Name/Maiden First Name M.I. Age Death Death/Birth/Residence Date, Page detailed obit Abbarno Vincent "Lolly" A. 9/26/2010 Kenmore, NY 9-30-2010: C4 Abbatte/Saunders Murielle A. 87 1/11/2010 1-13-2010: B4 Abbo Joseph D. 57 5/31/2010 Lewiston, NY 6-3-2010: B4 Brooksville, FL; formerly of Abbott Casimer "Casey" 12/19/22009 Cheektowaga, NY 4-18-2010: C6 Abbott Phillip C. 3/31/2010 4-3-2010: B4 Abbott Stephen E. 7/6/2010 7-8-2010: B4 Abbott/Pfoetsch Barbara J. 4/20/2010 5-2-2010: B4 Abeles Esther 95 1/31/2010 2-4-2010: C4 Abelson Gerald A. 82 2/1/2010 Buffalo, NY 2-3-2010: B4 Abraham Frank J. 94 3/21/2010 3-23-2010: B4 Abrahams/Gichtin Sonia 2/10/2010 died in California 2-14-2010: C4 Abramo Rafeala 93 12/16/2010 12-19-2010: C4 Abrams Charlotte 4/6/2010 4-8-2010: B4 Abrams S. "Michelle" M. 37 5/21/2010 Salamanca, NY 5-23-2010: B4 Abrams Walter I. 5/15/2010 Basom, NY 5-19-2010: B4 Abrosette/Aksterowicz Sister Mary 6/18/2010 6-19-2010: C4 Refer to BEN 2-21-2010: B6/7/8 for more possible Abshagen Charles, Jr. L. 73 2/19/2010 North Tonawanda, NY 2-22-2010: B8 information Acevedo Miguel A. 10/6/2010 Buffalo, NY 10-27-2010: B4 Achkar John E. -

Quarta-Feira, 15 De Maio De 2013
iário ficial Nº 10.627 - Ano XLIIID OPrefeitura Municipal de Campinas Quarta-feira, 15 de maio de 2013 www.campinas.sp.gov.br MÉRCIO E SAÚDE ANIMAL LTDA. EPP em relação à especifi cação do item 1 GABINETE DO PREFEITO descrita no Anexo I - Especifi cações dos Itens do edital, conforme segue: 1. A respeito das exigências do item 01, ração para cães fi lhotes, acreditamos que os níveis de garantia do alimento para cães fi lhotes devam ser maiores que os níveis DECRETO Nº 17.970 DE 14 DE MAIO DE 2013 de garantia do alimento para cães adultos. Normalmente o nível de proteína é de, no AUTORIZA DESCONTO AOS PROFESSORES E ESTUDANTES DAS RE- mínimo, 30% devido o animal em crescimento necessitar de mais nutrientes do que DES MUNICIPAL E ESTADUAL DE ENSINO NO PREÇO DO INGRES- um animal adulto. SO DOS CONCERTOS DA ORQUESTRA SINFÔNICA MUNICIPAL DE Resposta: Será disponibilizado novo Edital prevendo ração para cães fi lhotes com CAMPINAS E DÁ OUTRAS PROVIDÊNCIAS. PROTEÍNA BRUTA min. 32% e máx. 35%. O Prefeito do Município de Campinas, no uso de suas atribuições legais, Campinas, 14 de maio de 2013 DECRETA : JOAO FERNANDES FILHO Art. 1° Fica autorizado desconto aos professores e estudantes das Redes Municipal e Pregoeiro Estadual de Ensino no preço do ingresso dos concertos da Orquestra Sinfônica Mu- nicipal de Campinas que se realizarem nas dependências dos teatros e auditórios mu- EXPEDIENTE DESPACHADO PELO SR.SECRETÁRIO nicipais. MUNICIPAL DE ADMINISTRAÇÃO Art. 2º Para atendimento do disposto no art. 1º serão pagos os seguintes valores, por DECLARAÇÃO DE ITEM FRACASSADO E HOMOLOGAÇÃO ingresso: Processo Administrativo n º 12/10/56.360 I - 1/3 (um terço) do valor do ingresso para os professores; Interessado: Secretaria Municipal de Saúde II - 1/6 (um sexto) do valor do ingresso para os alunos. -

Targeted EDTA Chelation Therapy with Albumin Nanoparticles To
Clemson University TigerPrints All Dissertations Dissertations 8-2019 Targeted EDTA Chelation Therapy with Albumin Nanoparticles to Reverse Arterial Calcification and Restore Vascular Health in Chronic Kidney Disease Saketh Ram Karamched Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Karamched, Saketh Ram, "Targeted EDTA Chelation Therapy with Albumin Nanoparticles to Reverse Arterial Calcification and Restore Vascular Health in Chronic Kidney Disease" (2019). All Dissertations. 2479. https://tigerprints.clemson.edu/all_dissertations/2479 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. TARGETED EDTA CHELATION THERAPY WITH ALBUMIN NANOPARTICLES TO REVERSE ARTERIAL CALCIFICATION AND RESTORE VASCULAR HEALTH IN CHRONIC KIDNEY DISEASE A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Bioengineering by Saketh Ram Karamched August 2019 Accepted by: Dr. Narendra Vyavahare, Ph.D., Committee Chair Dr. Agneta Simionescu, Ph.D. Dr. Alexey Vertegel, Ph.D. Dr. Christopher G. Carsten, III, M.D. ABSTRACT Cardiovascular diseases (CVDs) are the leading cause of death globally. An estimated 17.9 million people died from CVDs in 2016, with ~840,000 of them in the United States alone. Traditional risk factors, such as smoking, hypertension, and diabetes, are well discussed. In recent years, chronic kidney disease (CKD) has emerged as a risk factor of equal importance. Patients with mild-to-moderate CKD are much more likely to develop and die from CVDs than progress to end-stage renal failure. -

International Research and Exchanges Board Records
International Research and Exchanges Board Records A Finding Aid to the Collection in the Library of Congress Prepared by Karen Linn Femia, Michael McElderry, and Karen Stuart with the assistance of Jeffery Bryson, Brian McGuire, Jewel McPherson, and Chanté Wilson-Flowers Manuscript Division Library of Congress Washington, D.C. 2011 International Research and Exchanges Board Records Page ii Collection Summary Title: International Research and Exchanges Board Records Span Dates: 1947-1991 (bulk 1956-1983) ID No: MSS80702 Creator: International Research and Exchanges Board Creator: Inter-University Committee on Travel Grants Extent: 331,000 items; 331 cartons; 397.2 linear feet Language: Collection material in English and Russian Repository: Manuscript Division, Library of Congress, Washington, D.C. Abstract: American service organization sponsoring scholarly exchange programs with the Soviet Union and Eastern Europe in the Cold War era. Correspondence, case files, subject files, reports, financial records, printed matter, and other records documenting participants’ personal experiences and research projects as well as the administrative operations, selection process, and collaborative projects of one of America’s principal academic exchange programs. International Research and Exchanges Board Records Page iii Contents Collection Summary .......................................................... ii Administrative Information ......................................................1 Organizational History..........................................................2 -

(12) Patent Application Publication (10) Pub. No.: US 2010/0222294 A1 Pele (43) Pub
US 2010O222294A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0222294 A1 Pele (43) Pub. Date: Sep. 2,9 2010 (54) FORMULATIONS OF ATP AND ANALOGS OF Publication Classification ATP (51) Int. Cl. A 6LX 3L/7076 (2006.01) (75) Inventor: Amir Pelleg, Haverford, PA (US) A6IP35/00 (2006.01) Correspondence Address: 39t. 87, C FSH & RICHARDSON P.C. (2006.01) P.O. BOX 1022 A6IP 9/00 308: MNNEAPOLIS. MN 55440-1022 US A6IP II/06 2006.O1 9 (US) A6IP II/08 (2006.01) (73) Assignee: DUSKA SCIENTIFIC CO., CI2N 5/02 (2006.01) Philadelphia, PA (US) AOIN I/02 (2006.01) (52) U.S. Cl. ................................ 514/47; 435/375; 435/2 (21) Appl. No.: 12/715,170 (57) ABSTRACT (22) Filed: Mar. 1, 2010 This disclosure provides solutions and compositions (e.g., O O pharmaceutical solutions and compositions) containing Related U.S. Application Data adenosine 5'-triphosphate (ATP) or an analog thereof. In (60) Provisional application No. 61/156.263, filed on Feb. addition, it features methods of making and using the solu 27, 2009. tions and compositions. Patent Application Publication Sep. 2, 2010 US 2010/0222294 A1 Figure , NH N O O. O. a'rn HO-P-O-PYo-E-40. O-P-O- o, 's-slNYN Ohi Oi O US 2010/0222294 A1 Sep. 2, 2010 FORMULATIONS OF ATP AND ANALOGS OF N-Tris(hydroxymethyl)methylglycine (Tricine); glycine; ATP Diglycine (Gly-Gly); N,N-Bis(2-hydroxyethyl)glycine (Bi cine); N-(2-Hydroxyethyl)piperazine-N'-(4-butanesulfonic acid) (HEPBS); N-Tris(hydroxymethyl)methyl-3-amino 0001. -

Biomedical Implications of Heavy Metals Induced Imbalances in Redox Systems
Hindawi Publishing Corporation BioMed Research International Volume 2014, Article ID 640754, 26 pages http://dx.doi.org/10.1155/2014/640754 Review Article Biomedical Implications of Heavy Metals Induced Imbalances in Redox Systems Bechan Sharma,1 Shweta Singh,2 and Nikhat J. Siddiqi3 1 Department of Biochemistry, University of Allahabad, Allahabad 211002, India 2 Department of Genetics, SGPGIMS, Lucknow 226014, India 3 Department of Biochemistry, King Saud University, Riyadh 11451, Saudi Arabia Correspondence should be addressed to Bechan Sharma; [email protected] Received 28 February 2014; Revised 28 May 2014; Accepted 10 July 2014; Published 12 August 2014 Academic Editor: Hartmut Jaeschke Copyright © 2014 Bechan Sharma et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Several workers have extensively worked out the metal induced toxicity and have reported the toxic and carcinogenic effects of metals in human and animals. It is well known that these metals play a crucial role in facilitating normal biological functions of cells as well. One of the major mechanisms associated with heavy metal toxicity has been attributed to generation of reactive oxygen and nitrogen species, which develops imbalance between the prooxidant elements and the antioxidants (reducing elements) in the body. In this process, a shift to the former is termed as oxidative stress. The oxidative stress mediated toxicity of heavy metals involves damage primarily to liver (hepatotoxicity), central nervous system (neurotoxicity), DNA (genotoxicity), and kidney (nephrotoxicity) in animals and humans. Heavy metals are reported to impact signaling cascade and associated factors leading to apoptosis. -

Transcriptomic Analysis of Synergy Between Antifungal Drugs and Iron Chelators for Alternative Antifungal Therapies
Transcriptomic analysis of synergy between antifungal drugs and iron chelators for alternative antifungal therapies Yu-Wen Lai School of Life and Environmental Sciences (SoLES) Faculty of Science The University of Sydney 2017 A thesis submitted in fulfilment of the requirements of the degree of Doctor of Philosophy. Declaration of originality I certify that this thesis contains no materials which have been accepted for award of any other degree or diploma at any other university. To the best of my knowledge, this thesis is original and contains no material previously published or written by any other person, except where due reference has been made in the text. Yu-Wen Lai August 2016 i Acknowledgements This whole thesis and the years spent into completing it would not have been possible without the help and support of a lot of people. I am thankful and grateful to my supervisor Dee Carter and also to my co-supervisor Sharon Chen for giving me the opportunity to work on this project. I am also thankful to our collaborator Marc Wilkins and his team. This project has been very challenging and your inputs and advice have really helped immensely. A big thank you goes to past and present honours students, PhD candidates and post-docs. Your prep talks, stimulating discussions, humour, baked goods and cider making sessions were my staples for keeping me mostly sane. To Kate Weatherby and Sam Cheung, we started honours and PhDs together and even travelled together in Europe. There were major ups and downs during the course of our projects, but were finally getting to the end! Thank you Katherine Pan and Mia Zeric for always offering chocolate and snacks, I swear I mostly go over to your side of the office just to eat your food.