Brown Shark with Horizontally Oval Eyes and Internal Nictitating
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sharks for the Aquarium and Considerations for Their Selection1 Alexis L
FA179 Sharks for the Aquarium and Considerations for Their Selection1 Alexis L. Morris, Elisa J. Livengood, and Frank A. Chapman2 Introduction The Lore of the Shark Sharks are magnificent animals and an exciting group Though it has been some 35 years since the shark in Steven of fishes. As a group, sharks, rays, and skates belong to Spielberg’s Jaws bit into its first unsuspecting ocean swim- the biological taxonomic class called Chondrichthyes, or mer and despite the fact that the risk of shark-bite is very cartilaginous fishes (elasmobranchs). The entire supporting small, fear of sharks still makes some people afraid to swim structure of these fish is composed primarily of cartilage in the ocean. (The chance of being struck by lightning is rather than bone. There are some 400 described species of greater than the chance of shark attack.) The most en- sharks, which come in all different sizes from the 40-foot- grained shark image that comes to a person’s mind is a giant long whale shark (Rhincodon typus) to the 2-foot-long conical snout lined with multiple rows of teeth efficient at marble catshark (Atelomycterus macleayi). tearing, chomping, or crushing prey, and those lifeless and staring eyes. The very adaptations that make sharks such Although sharks have been kept in public aquariums successful predators also make some people unnecessarily since the 1860s, advances in marine aquarium systems frightened of them. This is unfortunate, since sharks are technology and increased understanding of shark biology interesting creatures and much more than ill-perceived and husbandry now allow hobbyists to maintain and enjoy mindless eating machines. -

NPOA Sharks Booklet.Indd
National Plan of Action for the Conservation and Management of Sharks (NPOA-Sharks) November 2013 South Africa Department of Agriculture, Forestry and Fisheries Private Bag X2, Rogge Bay, 8012 Tel: 021 402 3911 Fax: +27 21 402 3364 www.daff.gov.za Design and Layout: FNP Communications and Gerald van Tonder Photographs courtesy of: Department of Agriculture, Forestry and Fisheries (DAFF), Craig Smith, Charlene da Silva, Rob Tarr Foreword South Africa’s Exclusive Economic Zone is endowed with a rich variety of marine living South Africa is signatory to the Code of Conduct for Responsible Fisheries – voluntarily agreed to by members of the United Nations Food and Agriculture Organisation (FAO) – and, as such, is committed to the development and implementation of National Plans of Action (NPOAs) as adopted by the twenty-third session of the FAO Committee on Fisheries in February 1999 and endorsed by the FAO Council in June 1999. Seabirds – aimed at reducing incidental catch and promoting the conservation of seabirds Fisheries and now regularly conducts Ecological Risk Assessments for all the commercial practices. Acknowledging the importance of maintaining a healthy marine ecosystem and the possibility of major detrimental effects due to the disappearance of large predators, South from the list of harvestable species. In accordance with international recommendations, South Africa subsequently banned the landing of a number of susceptible shark species, including oceanic whitetip, silky, thresher and hammerhead sharks. improves monitoring efforts for foreign vessels discharging shark products in its ports. To ensure long-term sustainability of valuable, but biologically limited, shark resources The NPOA-Sharks presented here formalises and streamlines ongoing efforts to improve conservation and management of sharks caught in South African waters. -

SUPPLEMENTARY ONLINE MATERIAL for New Specimen of the Rare Requiem Shark Eogaleus Bolcensis from the Bolca Lagerstätte, Italy G
http://app.pan.pl/SOM/app65-LaroccaConte_etal_SOM.pdf SUPPLEMENTARY ONLINE MATERIAL FOR New specimen of the rare requiem shark Eogaleus bolcensis from the Bolca Lagerstätte, Italy Gabriele Larocca Conte, Enrico Trevisani, Paolo Guaschi, and Federico Fanti Published in Acta Palaeontologica Polonica 2020 65 (3): 547-560. https://doi.org/10.4202/app.00725.2020 Supplementary Online Material SOM 1. Table 1. Measurements of Galeorhinus cuvieri and Eogaleus bolcensis. Table 2. Age estimates of Bolca specimens according to growth parameters of different extant populations of carcharhiniforms. SOM 2. Measurements of preserved teeth of MSNPV 24625 available at http://app.pan.pl/SOM/app65-LaroccaConte_etal_SOM/SOM_2.xlsx SOM 3. Counts and antero-posterior length of centra of Bolca carcharhiniforms assemblage available at http://app.pan.pl/SOM/app65-LaroccaConte_etal_SOM/SOM_3.xlsx References SOM 1. Table 1. Measurements (in mm) of Galeorhinus cuvieri and Eogaleus bolcensis. %TL = (X/TL) * 100; where %TL, percentage of the total length; X, length of the body segment. ID, morphometric measurement (see Fig. 1A for explanations). “+x” refers to the missing body fragment of the incomplete specimens. Galeorhinus cuvieri Eogaleus bolcensis MGP-PD 8869 C- ID MGP-PD 8871-8872 MCSNV T1124 MCSNV VIIB96-VIIB97 MGGC 1976 MNHN FBol516 MCSNV T311 8870 C cm %TL cm %TL cm %TL cm %TL cm %TL cm %TL cm %TL 1 69.4 1 92 1 83+x - 92 1 67+x - 135 1 - - 2 13.9 20.03 16 17.39 18 - 14.6 15.89 15.5+x - 23 17.04 - - 3 35.5 51.15 46 50 42 - 48.3 52.48 37 - 73 54.07 85.7 - 4 -

Sharks and Rays
SHARKS AND RAYS Photo by: © Jim Abernethy Transboundary Species - Content ... 31 32 33 34 35 ... Overview As stated in the previous section, the establishment of the Yarari fishing for sharks in the Netherlands and places new pressure on Marine Mammal and Shark Sanctuary was an important step fishermen to implement new techniques and updated fishing gear in protecting the shark and ray species of the Dutch Caribbean. to avoid accidentally catching sharks and rays as bycatch. Overall, there is a significant lack of information concerning these vital species within Dutch Caribbean waters. Fortunately, this There are several different international treaties and legisla- trend is changing and in the last few years there has been a push tion which offer protection to these species. This includes the to increase research, filling in the historic knowledge gap. Sharks Convention on International Trade in Endangered Species (CITES), and rays are difficult species to protect as they tend to have long the Specially Protected Areas and Wildlife (SPAW) protocol and reproduction cycles, varying between 3 and 30 years, small litters, the Convention on Migratory Species (CMS). Scientists are just which means they do not recover quickly when overfished and can beginning to uncover the complexities of managing conservation travel over great distances which makes them difficult to track. efforts for these species, as they often have long migration routes which put them in danger if international waters are not managed Early in 2019, the Ministry of Agriculture, Nature and Food Quality and protected equally. (LNV) published a strategy document to manage and protect sharks and rays within waters the Netherlands influences (this There are more than thirty different species of sharks and includes the North Sea, Dutch Caribbean and other international rays which are known to inhabit the waters around the Dutch waters). -

Latitudinal Disparity in the Reproductive Cycle of Sharpnose Shark, Rhizoprionodon Lalandii (Elasmobranchii: Carcharhinidae), in Atlantic Waters Off South America
ZOOLOGIA 29 (5): 413–419, October, 2012 doi: 10.1590/S1984-46702012000500004 Latitudinal disparity in the reproductive cycle of sharpnose shark, Rhizoprionodon lalandii (Elasmobranchii: Carcharhinidae), in Atlantic waters off South America Morgana M. Macedo1, Marcia F. Sousa1 & Vandick S. Batista1,2 1 Laboratório de Ecologia, Peixes e Pesca, Instituto de Ciências Biológicas e da Saúde, Universidade Federal de Alagoas, Campus A.C. Simões. Avenida Lourival Melo Mota, Tabuleiro do Martins, 57072-970 Maceió, AL, Brazil. 2 Corresponding author. E-mail: [email protected] ABSTRACT. Geographical variation in biophysical conditions may strongly influence the life history characteristics of widely distributed species, such as the Brazilian sharpnose shark, Rhizoprionodon lalandii (Müller & Henle, 1839). Here, we use original and secondary data of reproductive traits of R. lalandii to identify population differences among north- ern/northeastern and southern Atlantic waters of South America. In the southeast region, birth occurs between Decem- ber and March, and the young become frequent along the coast between April and September. Mating occurs mainly between March and June, when females with bite marks are common. Females in early pregnancy occur between March and September. The reproductive cycle of R. lalandii in the northern/northeastern region was approximately six months ahead of the cycle described for the southeastern region. These results support the hypothesis that environmen- tal conditions in the North-Northeast and Southeast generate differences in life history traits, resulting in at least two distinct populations along the Brazilian coast. KEY WORDS. Coastal waters; life history; reproductive traits; northeastern Brazil. Some ife history traits, such as life spans, are a pheno- productive cycle between these regions (LESSA 1988), suggest- typic expression of the interaction between genotype and the ing that these populations may be reproductively isolated. -

Integrating Multiple Chemical Tracers to Elucidate the Diet and Habitat of Cookiecutter Sharks Aaron B
www.nature.com/scientificreports OPEN Integrating multiple chemical tracers to elucidate the diet and habitat of Cookiecutter Sharks Aaron B. Carlisle1*, Elizabeth Andruszkiewicz Allan2,9, Sora L. Kim3, Lauren Meyer4,5, Jesse Port6, Stephen Scherrer7 & John O’Sullivan8 The Cookiecutter shark (Isistius brasiliensis) is an ectoparasitic, mesopelagic shark that is known for removing plugs of tissue from larger prey, including teleosts, chondrichthyans, cephalopods, and marine mammals. Although this species is widely distributed throughout the world’s tropical and subtropical oceanic waters, like many deep-water species, it remains very poorly understood due to its mesopelagic distribution. We used a suite of biochemical tracers, including stable isotope analysis (SIA), fatty acid analysis (FAA), and environmental DNA (eDNA), to investigate the trophic ecology of this species in the Central Pacifc around Hawaii. We found that large epipelagic prey constituted a relatively minor part of the overall diet. Surprisingly, small micronektonic and forage species (meso- and epipelagic) are the most important prey group for Cookiecutter sharks across the studied size range (17–43 cm total length), with larger mesopelagic species or species that exhibit diel vertical migration also being important prey. These results were consistent across all the tracer techniques employed. Our results indicate that Cookiecutter sharks play a unique role in pelagic food webs, feeding on prey ranging from the largest apex predators to small, low trophic level species, in particular those that overlap with the depth distribution of the sharks throughout the diel cycle. We also found evidence of a potential shift in diet and/or habitat with size and season. -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

View/Download
CARCHARHINIFORMES (Ground Sharks) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 32.0 - 17 June 2021 Order CARCHARHINIFORMES Ground Sharks 9 families · 54 genera/subgenera · 295 species/subspecies Family PENTANCHIDAE Deepwater Cat Sharks 11 genera · 111 species Apristurus Garman 1913 a-, not; pristis, saw; oura, tail, referring to absence of saw-toothed crest of enlarged dermal denticles along upper edge of caudal fin as found in the closely related Pristiurus (=Galeus) Apristurus albisoma Nakaya & Séret 1999 albus, white; soma, body, referring to whitish color Apristurus ampliceps Sasahara, Sato & Nakaya 2008 amplus, large; -ceps, head, which, apparently, it is Apristurus aphyodes Nakaya & Stehmann 1998 whitish, referring to pale gray coloration Apristurus australis Sato, Nakaya & Yorozu 2008 southern, referring to distribution in the southern hemisphere around Australia Apristurus breviventralis Kawauchi, Weigmann & Nakaya 2014 brevis, short; ventralis, of the belly, referring to very short abdomen Apristurus brunneus (Gilbert 1892) brown, referring to “uniform warm brown” color above and below Apristurus bucephalus White, Last & Pogonoski 2008 bu, large; cephalus, head, referring to large, broad head Apristurus canutus Springer & Heemstra 1979 hoary, referring to dark gray coloration with minute white spots underneath denticles Apristurus exsanguis Sato, Nakaya & Stewart 1999 bloodless or lifeless, referring to characteristic pale coloration and flaccid body Apristurus fedorovi Dolganov 1983 in honor -

Habitat Preference of Leopard Sharks (Triakis Semifasciata)
Habitat Preference of Leopard Sharks (Triakis semifasciata) at Chicago Zoological Society Based on Bottom Substrate Amanda (Williams) Flannery Miami University, Oxford, OH 2016 Chicago Zoological Society Cohort Abstract Much like several other species of near shore elasmobranchs, the leopard shark (Triakis semifasciata), relies on estuaries in the wild throughout their life histories to hide from predators, reproduce, and to use as pupping grounds and nurseries. However due to anthropogenic forces, these habitats have been subjected to development, pollution and agriculture which have led to destruction or alteration of nearly 90% of these environments along the Californian coastline. The objective of this study was to observe the Triakis semifasciata at Chicago Zoological Society in Brookfield, Illinois to determine how this social group of females use their habitat space based on bottom substrate. The sharks were observed for 6 days for 2 ½ hour periods in the morning (10:00am - 12:30pm) and early afternoon (12:30pm - 2:00pm) with a timer set to five minute intervals, at which point the position of each shark within the habitat was recorded. A behavioral ethogram was developed to capture behaviors relevant to habitat use. An ANOVA indicated there was statistical significance of habitat preference of sharks based on bottom substrate (F= 5.00, p= 0.049, F crit= 4.96), while a two-way ANOVA indicated there was no statistical significance between the time of observation and habitat bottom substrate preference by T.semifasciata females (F= 0.03, p= 0.84, F crit= 5.31). There was no statistical significance between the two observation periods and behaviors, as the standard errors overlapped significantly, indicating a great deal of variance in behaviors. -

(Carcharhinus Limbatus) in the Western Atlantic
Genetics and Molecular Biology, 35, 4, 752-760 (2012) Copyright © 2012, Sociedade Brasileira de Genética. Printed in Brazil www.sbg.org.br Research Article Inclusion of South American samples reveals new population structuring of the blacktip shark (Carcharhinus limbatus) in the western Atlantic Davidson Sodré1, Luis F.S. Rodrigues-Filho1, Rosália F.C. Souza2, Péricles S. Rêgo1, Horacio Schneider1, Iracilda Sampaio1 and Marcelo Vallinoto1,3 1Laboratório de Genética e Biologia Molecular, Instituto de Estudos Costeiros, Universidade Federal do Pará, Bragança, PA, Brazil. 2Universidade Federal Rural do Pará, Belém, PA, Brazil. 3Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Vairão, Portugal. Abstract Carcharhinus limbatus has a cosmopolitan distribution and marked genetic structuring, mainly because of its philopatric behavior. However, analysis of this structuring has not previously included South American populations. In the present study, we analyzed a sample of adult individuals collected on the northern coast of Brazil and com- pared the sequences of the mitochondrial control region with those of populations already genotyped. Relatively high haplotype diversity (12 haplotypes, genetic diversity of 0.796) was observed, similar to that in other populations but with a much larger number of private alleles. In contrast to populations studied previously, which were represented by neonates, the pronounced allelic variability found in the South American individuals may have resulted from mi- grations from other populations in the region that have yet to be genotyped. This population was also genetically dis- tinct from the other Atlantic populations (Fst > 0.8), probably because of female philopatry, and apparently separated from the northwestern Atlantic group 1.39 million years ago. -

Closing the Loopholes on Shark Finning
Threatened European sharks Like many animals before them, sharks have become prey to human indulgence. Today, sharks are among the ocean’s most threatened species. PORBEAGLE SHARK (Lamna nasus) BASKING SHARK (Cetorhinus maximus) COMMON THRESHER SHARK Similar to killing elephants for their valuable tusks, Critically Endangered off Europe Vulnerable globally (Alopias vulpinus) sharks are now often hunted for a very specific part of Closing Vulnerable globally their bodies – their fins. Fetching up to 500 Euros a kilo when dried, shark fins the SMOOTH HAMMERHEAD (Sphyrna zygaena) SPINY DOGFISH (Squalus acanthias) TOPE SHARK (Galeorhinus galeus) are rich pickings for fishermen. Most shark fins end up Endangered globally Critically Endangered off Europe Vulnerable globally in Asia where shark fin soup is a traditional delicacy and status symbol. loopholes With shark fins fetching such a high price, and with the rest of the shark being so much less valuable, many fishermen have taken to ‘finning’ the sharks they catch SHORTFIN MAKO (Isurus oxyrinchus) COMMON GUITARFISH (Rhinobatos rhinobatos) BLUE SHARK (Prionace glauca) Vulnerable globally Proposed endangered in Mediterranean Near Threatened globally to save room on their boats for the bodies of more on commercially important fish. shark GREAT WHITE SHARK (Carcharadon carcharias) COMMON SAWFISH (Pristis pristis) ANGEL SHARK (Squatina squatina) Vulnerable globally Assumed Extinct off Europe Critically Endangered off Europe finning Globally Threatened sharks on the IUCN (International Union -
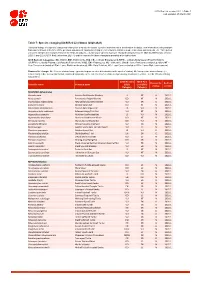
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-1: Table 7 Last Updated: 25 March 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category