Detection of Acute TLR-7 Agonist-Induced Hemorrhagic Myocarditis in Mice by Refined Multi-Parametric Quantitative Cardiac MRI
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non-Commercial Use Only
Veins and Lymphatics 2012; volume 1:e6 Ulcerated hemosiderinic three months previous to this therapy. Significant improvement in these injuries, Correspondence: Eugenio Brizzio and Alberto dyschromia and iron deposits with a reduction in the dimensions of the Lazarowski, San Martín 965, 1st floor (Zip code within lower limbs treated brown spot (9 of 9) at Day 90, and complete 1004) Buenos Aires, Argentina. with a topical application scarring with a closure time ranging from 15 Tel. +54.11.4311.5559. to 180 days (7 of 9) were observed. The use of E-mail: [email protected]; of biological chelator [email protected]; topical lactoferrin is a non-invasive therapeu- [email protected]; tic tool that favors clearance of hemosiderinic 1 2 [email protected] Eugenio Brizzio, Marcelo Castro, dyschromia and scarring of the ulcer. The 3 2 Marina Narbaitz, Natalia Borda, success of this study was not influenced Key words: ulcerated haemosiderinic dyschro- Claudio Carbia,2 Laura Correa,4 either by the hemochromatosis genetics or mia, liposomal Lactoferrin, scarring, hemo- Roberto Mengarelli,5 Amalia Merelli,2 the iron metabolism profile observed. siderin-ferritin. Valeria Brizzio,2 Maria Sosa,6 Acknowledgments: the authors would like to Biagio Biancardi,7 Alberto Lazarowski2 thank P. Girimonte for her assistance with the statistical analysis of the results. We also wish to Introduction thank the patients who made this study possible. 1International Group of Compression and Conference presentation: part of the present Argentina Medical Association, Buenos Chronic venous insufficiency (CVI) is one of study has been presented at the following con- 2 Aires, Argentina; Department of Clinical the most significant health problems in devel- gresses: i) XX Argentine Congress of Hematology, Biochemistry, Institute of oped countries. -

Simple Technique to Identify Haemosiderin in Immunoperoxidase Stained Sections
J Clin Pathol: first published as 10.1136/jcp.37.10.1190 on 1 October 1984. Downloaded from 1190 Technical methods Phosphate buffer at pH 8*0 gave the sharpest 2 Rozenszajn L, Leibovich M, Shoham D, Epstein J. The esterase staining reactions, although there was little differ- activity in megaloblasts, leukaemic and normal haemopoietic cells. Br J Haematol 1968; 14:605-19. ence at pH 7-0 or pH 7-5. As the buffer pH was 3Hayhoe FGJ, Quaglino D. Haematological cytochemistry. Edin- increased above pH 8-0 staining with both substrates burgh: Churchill Livingstone, 1980. became progressively weaker, especially above pH 4Li CY, Lam KW, Yam LT. Esterases in human leucocytes. J 9.0. Below pH 7-0 staining with a-naphthyl butyrate Histochem Cytochem 1973;21:1-12. Yam LT, Li CY, Crosby WH. Cytochemical identification of became weaker, and below pH 5*0 staining with monocytes and granulocytes. Am J Clin Pathol 1971;55:283- naphthol AS-D chloroacetate began to disappear. 90. 6 Armitage RJ, Linch DC, Worman CP, Cawley JC. The morphol- This work was supported by a Medical Research ogy and cytochemistry of human T-cell subpopulations defined by monoclonal antibodies and Fc receptors. Br J Haematol Council project grant. I thank Professor FGJ 1983;51:605-13. Hayhoe for valuable advice. References Requests for reprints to: Dr DM Swirsky, Department of Gomori G. Chloroacyl esters as histochemical substrates. J His- Haematological Medicine, University Clinical School, Hills tochem Cytochem 1953;1:469-70. Road, Cambridge CB2 2QL, England. Simple technique to identify identification of the two compounds on the same haemosiderin in slide. -

Gomori Prussian Blue Iron Stain Histology Staining Procedure
2505 Parview Road ● Middleton, WI 53562-2579 ● 800-383-7799 ● www.newcomersupply.com ● [email protected] July 2016 Gomori Prussian Blue Iron Stain - Technical Memo SOLUTIONS: 500 ml 1 Liter Hydrochloric Acid 20%, Aqueous Part 12087A Part 12087B Potassium Ferrocyanide 10%, Aqueous Part 13392A Additionally Needed: Iron Control Slides Part 4320 or Iron, Animal Control Slides Part 4321 Xylene, ACS Part 1445 Alcohol, Ethyl Denatured, 100% Part 10841 Alcohol, Ethyl Denatured, 95% Part 10842 Nuclear Fast Red Stain, Kernechtrot Part 1255 Hydrochloric Acid 5%, Aqueous Part 12086 (for acid cleaning glassware) For storage requirements and expiration date refer to individual product labels. APPLICATION: PROCEDURE NOTES: Newcomer Supply Gomori Prussian Blue Iron Stain is used to detect 1. Acid clean all glassware/plasticware (12086) and rinse thoroughly loosely bound ferric iron in tissue sections, bone marrow smears and in several changes of distilled water. Cleaning glassware with blood smears. This histochemical reaction is sensitive enough to bleach is not equivalent to acid washing. demonstrate even minute amounts of iron deposits in blood cells, bone 2. Drain staining rack/slides after each step to prevent solution carry marrow, spleen and liver. over. 3. Do not allow sections to dry out at any point during staining METHOD: procedure. Fixation: Formalin 10%, Phosphate Buffered (Part 1090) 4. Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid a. Chromate fixatives should be avoided cloudiness in dehydration steps. b. Fix smears per laboratory protocol 5. If using a xylene substitute, closely follow the manufacturer’s Technique: Paraffin sections cut at 5 microns or prepared smears recommendations for deparaffinization and clearing steps Solutions: All solutions are manufactured by Newcomer Supply, Inc. -

Supporting Information
Supporting Information Salazar et al. 10.1073/pnas.0804373105 SI Materials and Methods and postfixed for 24 h (4% paraformaldehyde and 0.1 M PBS). Chemicals and Antibodies. MPTP hydrochloride, 6-hydroxydopa- Alternatively, for TH cell counting in hemimesencephali, brains mine hydrobromide, Chelex 100, and D-amphetamine sulfate were postfixed for 48 h. Next, fixed brains were cryopreserved in were purchased from Sigma. Peptide N-glycosidase (PNGase) F 30% sucrose and cut on a freezing Microtome. Free-floating was purchased from New England Biolabs. DMT1 antibodies, sections were permeabilized, blocked for nonspecific binding previously developed and characterized (1), were used to rec- sites, and incubated with primary antibodies. Immunolabeling ognize the ϩIRE C-terminal region, the ϪIRE C-terminal was visualized using Alexa 488- and Cy3-conjugated secondary region, the 4th extracellular domain (Alpha Diagnostics), and antibodies (Invitrogen and Jackson ImmunoResearch, respec- the 3rd extracellular domain (Biosonda). In addition, the fol- tively) or HRP-conjugated secondary antibodies and DAB rev- lowing antibodies were used: rabbit anti-TH (Pel-Freez Biologi- elation (Vector Laboratories). cals), mouse anti-TH (Diasorin), rat anti-CD11b/Mac1 (Sero- tec), and mouse anti-actin (Sigma). Animals. Male C57BL/6J mice 12 weeks old were obtained from Janvier Breeding Center. Microcytic mice (MK/ReJ-mk/ϩ) were Tissue Preparation for Iron Measurement and Western Blot Analysis. obtained from Funmei Yang, University of Texas Health Sci- Within2hafterautopsy, the brains were dissected and blocks of ences Center, San Antonio, TX, and Mark Fleming, Harvard hemibrainstem were frozen in dry ice and stored at Ϫ80 °C. University School of Medicine, Boston, MA, and subsequently Serial 20-m-thick sections were cut from the frozen blocks at maintained as an inbred stock by breeding heterozygotes in a Ϫ12 °C by using a cryostat, thaw-mounted onto gelatin/ 129sv background. -

Malakoplakia of the Gastrointestinal Tract JOHN MCCLURE B.Sc., M.D., M.R.C.Path., D.M.J.Path
Postgrad Med J: first published as 10.1136/pgmj.57.664.95 on 1 February 1981. Downloaded from Postgraduate Medical Journal (February 1981) 57, 95-103 Malakoplakia of the gastrointestinal tract JOHN MCCLURE B.Sc., M.D., M.R.C.Path., D.M.J.Path. Division of Tissue Pathology, Institute ofMedical and Veterinary Science, Frome Road, Adelaide, South Australia, 5000 Summary sought medical aid for frequent loose bowel actions. The clinical and pathological features of 3 cases of There were no other symptoms and no significant colonic malakoplakia are documented thereby bringing past medical history. Sigmoidoscopy revealed an to 34 the total of recorded cases of malakoplakia ulcerated tumour 70 mm above the anus. A biopsy involving the gastrointestinal tract. This is therefore confirmed the presence of an adenocarcinoma of the most common site of involvement outside the colonic origin. Subsequently an abdomino-perineal urogenital tract. A comprehensive review of the world excision of the rectum was performed and the patient literature on gastrointestinal malakoplakia has been is alive and well 2 years after this operation. made and the characteristic features of the condition The resected specimen was a segment of rectum have been delineated. There was a bimodal age and anus 320 mm in length. There was a fungating Protected by copyright. incidence with a small cluster of cases occurring in tumour 60 mm in diameter and 30 mm in height and childhood and associated with significant additional situated 100 mm from the distal margin. The tumour systemic disease. In the adult cases the average age extended into the bowel wall and there were numer- was 57 years with a slight excess of males. -
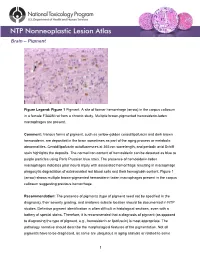
Brain – Pigment
Brain – Pigment Figure Legend: Figure 1 Pigment. A site of former hemorrhage (arrow) in the corpus callosum in a female F344/N rat from a chronic study. Multiple brown-pigmented hemosiderin-laden macrophages are present. Comment: Various forms of pigment, such as yellow-golden ceroid/lipofuscin and dark brown hemosiderin, are deposited in the brain sometimes as part of the aging process or metabolic abnormalities. Ceroid/lipofuscin autofluoresces at 365 nm wavelength, and periodic acid Schiff stain highlights the deposits. The normal iron content of hemosiderin can be detected as blue to purple particles using Perls Prussian blue stain. The presence of hemosiderin-laden macrophages indicates prior neural injury with associated hemorrhage resulting in macrophage phagocytic degradation of extravasated red blood cells and their hemoglobin content. Figure 1 (arrow) shows multiple brown-pigmented hemosiderin-laden macrophages present in the corpus callosum suggesting previous hemorrhage. Recommendation: The presence of pigments (type of pigment need not be specified in the diagnosis), their severity grading, and anatomic subsite location should be documented in NTP studies. Definitive pigment identification is often difficult in histological sections, even with a battery of special stains. Therefore, it is recommended that a diagnosis of pigment (as opposed to diagnosing the type of pigment, e.g., hemosiderin or lipofuscin) is most appropriate. The pathology narrative should describe the morphological features of the pigmentation. Not all pigments have to be diagnosed, as some are ubiquitous in aging animals or related to some 1 Brain – Pigment other disease process and are not toxicologically meaningful. The pathologist should use his or her judgment in deciding whether or not secondary deposits of pigment are prominent enough to warrant a separate diagnosis. -

Prussian Blue - Nuclear Fast Red Staining Kit
Prussian Blue - nuclear fast red staining kit Cat.No.:E670110 Package:100 Test /500 Test Description Hemoglobin (hemosiderin) is a hemoglobin-derived pigment, golden yellow or brown particles, because of its iron, golden yellow, it is called hemosiderin. When red blood cells are phagocytosed by macrophages, hemoglobin is broken down into iron-free orange blood and iron-containing hemosiderin under the action of lysosomal enzymes. Perls Prussian blue reaction (Prussian blue reaction), also known as hemosiderin staining, that is, after treatment of potassium ferrocyanide and dilute acid can produce blue, common in phagocytes or interstitial, the main display of ferric salt. Perls Prussian blue is a very classical histochemical reaction that is a sensitive, traditional and excellent method of displaying ferric iron in tissues. The staining principle is that potassium ferrocyanide makes the ferric ion from the protein diluted with hydrochloric acid Isolated, ferric iron and potassium ferrocyanide reaction to generate a blue insoluble compound that ferric ferricyanide Prussian blue, so the reaction is called Prussian blue reaction. Ferrous iron ferrocyanide is a very stable compound, after the reaction can be red dye staining, such as nuclear red, eosin, such as neutral red. Prussian blue - nuclear red staining kit commonly used to show that various local hemorrhagic lesions within the organization, common in phagocytes. In determining the hemosiderin deposition, Perls reaction can be confirmed, the staining method can be a good distinction between hemosiderin and other pigments. The dyeing liquid has good stability, can be stored for a long time, is not easy to produce precipitation, has a wide range of applications, can be counterstained. -

Prussian Blue - Eosin Staining Kit
Prussian blue - Eosin staining kit Cat.No.:E670108 Package:100 Test /500 Test Description Hemoglobin (hemosiderin) is a hemoglobin-derived pigment, golden yellow or brown particles, because of its iron, golden yellow, it is called hemosiderin. When red blood cells are phagocytosed by macrophages, hemoglobin is broken down into iron-free orange blood and iron-containing hemosiderin under the action of lysosomal enzymes. Perls Prussian blue reaction (Prussian blue reaction), also known as hemosiderin staining, that is, after treatment of potassium ferrocyanide and dilute acid can produce blue, common in phagocytes or interstitial, the main display of ferric salt. Perls Prussian blue is a very classical histochemical reaction that is a sensitive, traditional and excellent method of displaying ferric iron in tissues. The staining principle is that potassium ferrocyanide makes the ferric ion from the protein diluted with hydrochloric acid Isolated, ferric iron and potassium ferrocyanide reaction to generate a blue insoluble compound that ferric ferricyanide Prussian blue, so the reaction is called Prussian blue reaction. Ferrous iron ferrocyanide is a very stable compound, after the reaction can be red dye staining, such as nuclear red, eosin, such as neutral red. Prussian blue staining reagents commonly used to show a variety of hemorrhagic lesions within the local tissue, common in phagocytes. In determining the hemosiderin deposition, Perls reaction can be confirmed, the staining method can be a good distinction between hemosiderin and other pigments. The dyeing liquid has good stability, can be stored for a long time, is not easy to produce precipitation, has a wide range of applications, can be counterstained. -

Dye Origins Hematoxylin Is Extracted from the Logwood Tree and Purified. It Is Then Oxidized and Combined with a Mordant (Typica
Dye Origins Hematoxylin is extracted from the logwood tree and purified. It is then oxidized and combined with a mordant (typically aluminum) to allow it to bind to the cell structures. Of the many hematoxylin preparations used in histology Gill’s hematoxylin, Harris's hematoxylin and Mayer's hematoxylin are the most popular. Eosin is formed by a reaction between bromine and fluorescein. There are two eosin variants typically used in histology: eosin Y which is slightly yellowish and eosin B which is slightly bluish. Eosin Y is most popular. Special Stains The term special stains traditionally referred to any staining other than an H&E. It covers a wide variety of methods that may be used to visualize particular tissue structures, elements, or even microorganisms not identified by H&E staining. Other methods of staining use immunohistochemistry or in situ hybridization to target specific proteins or DNA/RNA sequences. These methods were sometimes also included as members of the “special stains” family. However, they are quite different in method and purpose and are now typically separated into a third category known as “advanced stains”. While there are literally hundreds of special stains for all manner of purposes, only a few are used with any regularity in clinical histology. The variety of stains also means that special staining is not as automated as H&E staining. While many larger laboratories do use automated instruments for the more common stains, they still have an area for hand staining. The complexity of some stains also works against the uses of automation. Some Common Special Stains. -

Histologic® Celebrates Its Reviewed Predictions That Had Been Have Not Changed Much; Microtomy 30Th Anniversary! Over the Years It Made for the 1970-1980 Decade
HISTOLOGIC ® T echnical Bulletin for Histotechnology Vol. XXXIV, No. 2, Nov. 2001 it has made a difference in spread- web site at www.sakuraus.com. 30 Years and Still ing valuable information to histo- It is interesting to realize that some Going Strong technologists and pathologists of the same conclusions made then around the world. also apply today. Faster, more Vinnie Della Speranza automated processing systems are Scientific Editor In 1981 for this publication’s 10th not yet here; automated stainers are anniversary, Lee G. Luna, editor, now widely used, but their principles This year HistoLogic® celebrates its reviewed predictions that had been have not changed much; microtomy 30th anniversary! Over the years it made for the 1970-1980 decade. has not evolved significantly except has been read by close to 1,000,000 The original article can be retrieved for the more widespread use of people worldwide. We believe that from the Sakura Finetek U.S.A. motorized units. 21 There have been advances, how- times are critical in advancing ever, with automated coverslippers. The Golden Age technology and histologic technique. For example, by using the Tissue- Barry R. J. Rittman, PhD Tek ® SCA™ Film Coverslipper, Much of the work being carried out slides can be viewed sooner under My perspective is largely colored today is based on the discoveries the microscope, thanks to the fast by my training as a medical labora- made in the 18th and 19th centuries. drying time. The fact that it cover- tory technologist and histologist in It was not until the 1820s that slips one slide every 3 seconds also England and the United States, microscope lenses which corrected helps! Slide labeling now can be including my training in electron for chromatic aberration were being done without using paper labels manufactured, usually on a custom microscopy and image analysis, and 1 and practical bar-coding is on the my teaching in histology and basis. -

Obesity Alters Adipose Tissue Macrophage Iron Content and Tissue Iron Distribution
Diabetes Volume 63, February 2014 421 Jeb S. Orr,1 Arion Kennedy,1 Emily K. Anderson-Baucum,1 Corey D. Webb,1 Steve C. Fordahl,2 Keith M. Erikson,2 Yaofang Zhang,3 Anders Etzerodt,4 Søren K. Moestrup,4 and Alyssa H. Hasty1 Obesity Alters Adipose Tissue Macrophage Iron Content and Tissue Iron Distribution Adipose tissue (AT) expansion is accompanied by contribute to the observed changes in MFehi ATM the infiltration and accumulation of AT macrophages phenotype and their reduced capacity to handle iron. (ATMs), as well as a shift in ATM polarization. Diabetes 2014;63:421–432 | DOI: 10.2337/db13-0213 Several studies have implicated recruited M1 ATMs in the metabolic consequences of obesity; however, METABOLISM little is known regarding the role of alternatively Obesity is marked by the preferential accumulation of activated resident M2 ATMs in AT homeostasis or inflammatory M1 adipose tissue (AT) macrophages how their function is altered in obesity. Herein, we (ATMs), which play an important role in the report the discovery of a population of alternatively development of AT inflammation and insulin resistance activated ATMs with elevated cellular iron content (IR) (1). The onset of AT dysfunction has important and an iron-recycling gene expression profile. These implications systemically, as AT inflammation and iron-rich ATMs are referred to as MFehi, and the dysregulated lipolysis both promote ectopic lipid remaining ATMs are referred to as MFelo. In lean deposition and the accompanying metabolic mice, ~25% of the ATMs are MFehi; this percentage consequences (2). Not surprisingly, a vast majority of the decreases in obesity owing to the recruitment of current literature is focused on mechanisms contributing MFelo macrophages. -

Combined Silver Perls's Stain for Differential
766 Y Clin Pathol 1993;46:766-768 Combined silver Perls's stain for differential staining of ringed sideroblasts and marrow iron J Clin Pathol: first published as 10.1136/jcp.46.8.766 on 1 August 1993. Downloaded from K T Tham, J B Cousar Abstract tain ringed sideroblasts were also included in During a study of nucleolar organiser the study. Three were from patients with regions, a modified silver stain was found myelodysplastic syndrome, and two from to be a sensitive marker for the iron in patients with erythroleukaemia and acute ringed sideroblasts, more so than Perls's lymphocytic leukaemia, respectively. Paraffin stain when the marrow iron stores were wax sections were stained with a Mallory low. To enhance the usefulness of the sil- modification of Perls's stain and also with the ver stain, a combined silver Perls CSP silver and Perls's stain and examined method was developed. This stains the microscopically for ringed sideroblasts. ringed sideroblast iron black and hae- mosiderin blue, thus rendering the PROCEDURE FOR THE COMBINED SILVER AND detection of ringed sideroblasts easier PERLS'S STAIN even when marrow iron stores are exces- 1 Dewax paraffin sections and bring down sive. At the same time, it allows marrow to water with decreasing concentrations of iron content to be evaluated. The silver ethanol. reagent in this combined method proba- 2 If the marrow tissue is fixed in fixative bly shows phosphate rather than the iron containing mercury, remove mercury with present in the abnormal mitochondria in Lugol's iodine for 5 minutes. Wash sections ringed sideroblasts.