Special Lecture Oxazaphosphorine Cytostatics: Past-Present-Future Seventh Cain Memorial Award Lecture1
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Anticancer Alkylating Agents-Part-I.Pdf
Anticancer Agents Medicinal Chemistry III B Pharm Dr. bilal al-jaidi Assistant Professor, Pharmaceutical Medicinal Chemistry Faculty of Pharmacy, Philadelphia University-Jordan Email: [email protected] Learning Outcome At the end of this lesson students will be able to – Outline the current status, causes and treatment strategies of cancer – Explain the mechanism of action, SAR, therapeutic uses and side effects of following classes of anti-cancer agents: • Alkylating Agents QUIZ = 20 Marks • Heavy metal compounds (Metallating Agents) • Anti-metabolite • Antibiotics • Plant Extracts • Topoisomerase inhibitors • Hormones • Combination of chemotherapy with other treatments • Others First Examination= 20 Marks The Status of Cancer • Cancer is a leading cause of death worldwide, accounting for 12.6 million new cases and 7.6 million deaths every year. THIS IS EQUIVALENT TO ONE PERSON, EVERY 5 SECONDS OF EVERYDAY Source: GLOBOCAN 2008 By 2020 the World Health Organisation (WHO) expects this rise to 16 million. Cancer • A new growth of tissue in which multiplication of cells is uncontrolled and progressive (tumour). • Abnormal cells can spread to other parts of the body (metastasise). Cancer Types Cancer types are categorized based on the functions/locations of the cells from which they originate: Carcinoma: a tumor derived from epithelial cells, those cells that line the inner or outer surfaces of our skin and organs (80-90% of all cancer cases reported) Sarcoma: a tumor derived from muscle, bone, cartilage, fat or connective tissues. Leukemia: a cancer derived from white blood cells or their precursors. Lymphoma: a cancer of bone marrow derived cells that affects the lymphatic system. Myelomas: a cancer involving the white blood cells responsible for the production of antibodies (B lymphocytes). -
![Chlormethine Gel for Treating Mycosis Fungoides-Type Cutaneous T- Cell Lymphoma [ID1589]](https://docslib.b-cdn.net/cover/7053/chlormethine-gel-for-treating-mycosis-fungoides-type-cutaneous-t-cell-lymphoma-id1589-1067053.webp)
Chlormethine Gel for Treating Mycosis Fungoides-Type Cutaneous T- Cell Lymphoma [ID1589]
Chlormethine gel for treating mycosis fungoides-type cutaneous T- cell lymphoma [ID1589] Produced by Aberdeen HTA Group Authors Dwayne Boyers1 Elisabet Jacobsen1 Clare Robertson3 Mari Imamura3 Dolapo Ayansina2 Paul Manson3 Gavin Preston4 Miriam Brazzelli3 1 Health Economics Research Unit, University of Aberdeen, UK 2 Medical Statistics Team, University of Aberdeen, UK 3 Health Services Research Unit, University of Aberdeen, UK 4 NHS Grampian, Aberdeen Royal Infirmary, Aberdeen, UK Correspondence to Miriam Brazzelli, Reader (Research) University of Aberdeen, Health Services Research Unit Foresterhill, Aberdeen, AB25 2ZD Email: [email protected] Date completed: 14 April 2020 Version: 2.0 (revised after factual error check) Copyright belongs to University of Aberdeen HTA Group, unless otherwise stated. : Copyright 2020 Queen's Printer and Controller of HMSO. All rights reserved. Source of funding: This report was commissioned by the NIHR Systematic Reviews Programme as project number 130927. Declared competing interests of the authors No competing interests to disclose. Acknowledgements The authors are grateful to Lara Kemp for providing administrative support. Copyright is retained by Recordati Rare Diseases/Helsinn Healthcare SA for Figures 1, 2, 3, 4, 5 and 6, and Table 8. Rider on responsibility for report The view expressed in this report are those of the authors and not necessarily those of the NIHR HTA Programme. Any errors are the responsibility of the authors. This report should be referenced as follows: Boyers D, Jacobsen E, Robertson C, Imamura M, Ayansina D, Manson P, Preston G, Brazzelli M. Chlormethine gel for treating mycosis fungoides-type cutaneous T-cell lymphoma. Aberdeen HTA Group, 2020. -

Purging and Haemopoietic Progenitor Cell Selection by CD34 Cell Separation
Bone Marrow Transplantation, (1998) 21, 665–671 1998 Stockton Press All rights reserved 0268–3369/98 $12.00 Purging and haemopoietic progenitor cell selection by CD34؉ cell separation ¨ ¨ W Kruger1, M Gruber1, S Hennings1, N Fehse1, B Fehse1, K Gutensohn2, N Kroger1 and AR Zander1 1Bone Marrow Transplantation Unit, 2Blood Transfusion Service, University Hospital Eppendorf, Hamburg, Germany Summary: High-dose chemotherapy supported by autologous stem cell reinfusion for treatment of metastatic and high-risk female breast cancer is currently under investigation in several Tumour cell contamination of autologous peripheral American and European studies.1,2,3 Tumour cell contami- blood stem cell samples (PBSC) and bone marrow (BM) nation of autologous stem cell products has been described is frequent. Enrichment of CD34+ stem cells is a promis- using immunocytochemistry, reverse transcriptase PCR and ing approach to purging tumour cells from autografts cell culture techniques in approximately one third of har- without damaging progenitor cells. Breast cancer cells vests with a range from 0% to 100%.4–7 The clonogenic were seeded (10؊3؊10؊7) into mononuclear cells from and metastatic potential of tumour cells isolated from blood G-CSF-mobilised PBSC and BM harvests from patients and autografts was demonstrated in cell culture assays and without breast cancer. CD34+ cells were enriched from in nude mice.8–10 The metastatic potential of accidentally mixtures either by immunomagnetic separation (Isolex- retransplanted tumour cells could be investigated by gene 50, and MiniMACS) or by biotin-streptavidin immu- marking of autografts prior to infusion followed by the noaffinity columns (Ceprate-LC). CD34؉ cell fractions detection of marked cells after relapse.11 However, due to were determined by FACS, cancer cells were detected the poor transduction efficacy of epithelial cancer cells by immunocytochemically with an anti-pancytokeratin gene transfer, a negative result would not exclude the possi- antibody. -

Rtc-Fertility-Preservation-280318
Fertility Preservation Oncofertility, social egg freezing and the transgender experience Dr Tamara Hunter FRANZCOG CREI Gynaecologist and Fertility Subspecialist Fertility Preservation • Oncofertility – Chemotherapy – Radiotherapy • Fertility preservation – Female – Male • Transgender fertility preservation • Elective oocyte cryopreservation (social egg freezing Oncofertility • 10% of cancers occur in women under 45yo • 50% have gonadotoxic treatment • 83% survive • Treatment, not disease itself premature ovarian failure – Chemotherapy – Radiotherapy • Loss of fertility • Menopause related complications Gonadotoxicity Risk of gondal dysfunction Sonmezer & Oktay 2004 High risk Medium risk Low risk • Cyclophosphamide • Cisplatin •Vincristine • Ifosfamide • Carboplatin •Methotrexate • Chlormethine • Doxorubicin •Bleomycin • Dactinomycin • Busulfan •Mercaptopurine •Vinblastine • Melphalan • Procarbazine • Chlorambucil Cyclophosphamide will induce permanent amenorrhea in high doses dependent on age 5g at age 40 10g at age 30 20g at age 20 Koyama et al. 1977 Risks of radiotherapy Impact depends on; –field of treatment –dose of radiation –fractionation Risks of radiotherapy • Estimated dose at which half oocytes depleted is 2Gy. • At birth 20 Gy to ovaries will cause ovarian failure • Women <30 15 Gy • Women <40 14 Gy • Women >40 6Gy (Hamish et al. 2005) Risk of radiotherapy In addition; • CNS irradiation may lead to hypogonadotrophic hypogonadism Uterine effects of radiotherapy • Decreased endometrial vascularisation • Decreased endometrial -

Chlormethine Hydrochloride (Brand Name: Ledaga®)
www.scottishmedicines.org.uk Decision Explained Medicine: chlormethine hydrochloride (brand name: Ledaga®) Recordati Rare Diseases UK Ltd The Scottish Medicines Consortium (SMC) has assessed chlormethine hydrochloride for the topical treatment (treatment that is applied on the skin) of adults with mycosis fungoides cutaneous T-cell Pharmalymphoma Company (MF-CTCL). This document summarises the SMC decision and what it means for patients. What has SMC said? After careful consideration, SMC has accepted chlormethine hydrochloride for the treatment of MF- CTCL as described above. This SMC advice takes into account a confidential discount offered by the pharmaceutical company that improves the cost-effectiveness of chlormethine hydrochloride. In addition, SMC was able to apply a more flexible approach* in the assessment, as it is for a rare condition. What does SMC’s decision mean for patients? If your healthcare professional thinks that chlormethine hydrochloride for use as described above is the right medicine for you, you should be able to have the treatment on the NHS in Scotland. What is chlormethine hydrochloride used for? Chlormethine hydrochloride is used to treat MF-CTCL. MF-CTCL is a type of cancer where the T-cells (cells of the body’s immune system) in the skin grow in an uncontrolled way. This leads to a build-up of abnormal T-cells, which can cause oval or ring-like patches and plaques to form on the skin. These can cause pain and itching, which may be severe. MF-CTCL develops over a long period of time. It is the most common form of a group of cancers known as CTCLs, which are long-term cancers of cells in the immune system that affect the skin. -

Ep 2962731 A1
(19) TZZ ¥__T (11) EP 2 962 731 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 06.01.2016 Bulletin 2016/01 A61P 35/00 (2006.01) A61K 45/06 (2006.01) A61K 39/395 (2006.01) A61K 31/337 (2006.01) (21) Application number: 15165855.6 (22) Date of filing: 07.01.2009 (84) Designated Contracting States: (72) Inventor: Jure-Kunkel, Maria AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Princton, NJ New Jersey 08543 (US) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK TR (74) Representative: Reitstötter Kinzebach Patentanwälte (30) Priority: 08.01.2008 US 19778 Sternwartstrasse 4 29.05.2008 US 56957 81679 München (DE) (62) Document number(s) of the earlier application(s) in Remarks: accordance with Art. 76 EPC: This application was filed on 30-04-2015 as a 09701364.3 / 2 227 296 divisional application to the application mentioned under INID code 62. (71) Applicant: Bristol-Myers Squibb Company Princeton, NJ 08543 (US) (54) COMBINATION OF IPILIMUMAB AND PACLITAXEL FOR THE TREATMENT OF CANCER (57) Compositions and methods are disclosed which are useful of the treatment and prevention of proliferative disorders. EP 2 962 731 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 962 731 A1 Description FIELD OF THE INVENTION 5 [0001] This invention relates to the fields of oncology and improved therapy regimens. BACKGROUND OF THE INVENTION [0002] The National Cancer Institute has estimated that in the United States alone, 1 in 3 people will be struck with 10 cancer during their lifetime. -

Other Alkylating Agents
Anticancer agents The Alkylating agents The alkylating agents are the oldest group of cytostatic drugs. The first of them – mechlorethamine was introduced into therapy in 1949. The alkylating agents can contain one or two alkylating groups. In the body the alkylating agents may react with DNA, RNA and proteins, although their reaction with DNA is believed to be the most important. 1 • The primary target of DNA coross-linking agents is the actively dividing DNA molecule. • The DNA cross-linkers are all extremly reactive nucleophilic structures (+). • When encountered, the nucleophilic groups on various DNA bases (particularly, but not exclusively, the N7 of guaninę) readily attack the electrophilic drud, resulting in irreversible alkylation or complexation of the DNA base. • Some DNA alkylating agents, such as the nitrogen mustards and nitrosoureas, are bifunctional, meaning that one molecule of the drug can bind two distinct DNA bases. 2 • Most commonly, the alkylated bases are on different DNA molecules, and intrastrand DNA cross-linking through two guaninę N7 atoms results. • The DNA alkylating antineoplastics are not cel cycle specific, but they are more toxic to cells in the late G1 or S phases of the cycle. • This is a time when DNA unwinding and exposing its nucleotides, increasing the Chance that vulnerable DNA functional groups will encounter the nucleophilic antineoplastic drug and launch the nucleophilic attack that leads to its own destruction. • The DNA alkylators have a great capacity for inducing toth mutagenesis and carcinogenesis, they can promote caner in addition to treating it. 3 • Organometallic antineoplastics – Platinum coordination complexes, also cross-link DNA, and many do so by binding to adjacent guanine nucleotides, called disguanosine dinucleotides, on the single strand of DNA. -

Final Scope PDF 169 KB
Appendix B NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Chlormethine gel for treating mycosis fungoides-type cutaneous T-cell lymphoma [ID1589] Final scope Draft remit/appraisal objective To appraise the clinical and cost effectiveness of chlormethine gel within its marketing authorisation for treating mycosis fungoides-type cutaneous T-cell lymphoma. Background Lymphomas are cancers of the lymphatic system. They are broadly divided into Hodgkin’s and non-Hodgkin’s lymphomas. Cutaneous T-cell lymphoma is a rare type of non-Hodgkin’s lymphoma that affects the skin. It is caused by the uncontrolled growth of T-lymphocytes within the skin. Many types of cutaneous T-cell lymphoma start as flat, scaly, oval patches or plaques on the skin, which progress to skin tumours, and are often itchy and sometimes painful. Some people with cutaneous T-cell lymphoma experience swelling of the lymph nodes. Within the group of cutaneous T-cell lymphoma, there are distinct subtypes. Mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It is usually a very slow-growing type of lymphoma that often only affects the skin and can stay under control for many years. In England in 2017, there were around 12,065 new cases of non-Hodgkin’s lymphomas and 796 people had a primary diagnosis of peripheral or cutaneous T-cell lymphoma1 There were 107 men and 72 women diagnosed with mycosis fungoides cutaneous T-cell lymphoma, in England in 2017.1 Median survival with early-stage disease, stage IA, IB and IIA, is reported as 35.5, 21.5 and 15.8 years, respectively. -

Cytotoxic and Other Chemotherapeutic Agents
Procedure for the Safe Prescribing, Handling and Administration of Cytotoxic and other Chemotherapeutic Agents CATEGORY: Procedural Document CLASSIFICATION: Clinical PURPOSE This document explains the procedures to be taken by clinical staff within University Hospitals Birmingham NHS Foundation Trust for the safe prescribing, handling and administration of cytotoxic and other chemotherapeutic agents Controlled Document 504 Number: Version Number: 3 DOCUMENT Controlled Document Executive Medical Director Sponsor: Executive Chief Nurse Director of Pharmacy Controlled Document Lead Chemotherapy Nurse Lead: Lead Cancer Services Pharmacist Approved By: MMAG On: December 2014 Review Date: December 2017 Distribution: All Clinical Staff within • Essential University Hospitals Birmingham Reading for: NHS Foundation Trust who are responsible for the Safe CONTROLLED Prescribing, Handling and Administration of Cytotoxic and other Chemotherapeutic Agents • Information for: All Clinical Staff Doc Index No. 504 Version 3 Page 1 of 29 Procedure for the safe prescribing, handling and administration of cytotoxic and other chemotherapeutic agents UNIVERSITY HOSPITALS BIRMINGHAM NHS FOUNDATION TRUST Procedure for the Safe Prescribing, Handling and Administration of Cytotoxic and other Chemotherapeutic Agents Contents Page 1.0 Introduction and aim of the document 5 2.0 Definition 5 2.1 Cytotoxic 5 2.2 Chemotherapy and Chemotherapeutic Agents 6 2.3 Non-cancer cytotoxic drugs used in other clinical settings 6 2.4 Monoclonal Antibodies, fusion proteins and other -
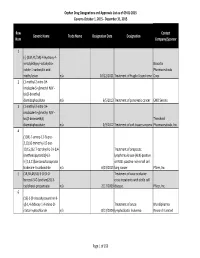
Orphan Drug Designation List
Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 1 (‐)‐(3aR,4S,7aR)‐4‐Hydroxy‐4‐ m‐tolylethynyl‐octahydro‐ Novartis indole‐1‐carboxylic acid Pharmaceuticals methyl ester n/a 10/12/2011 Treatment of Fragile X syndrome Corp. 2 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐broethyl) diamidophosphate n/a 6/5/2013 Treatment of pancreatic cancer EMD Serono 3 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐bromoethyl) Threshold diamidophosphate n/a 3/9/2012 Treatment of soft tissue sarcoma Pharmaceuticals, Inc. 4 (1OR)‐7‐amino‐12‐fluoro‐ 2,10,16‐trimethyl‐15 oxo‐ 10,15,16,17‐tetrahydro‐2H‐8,4‐ Treatment of anaplastic (metheno)pyrazolo[4,3‐ lymphoma kinase (ALK)‐positive h][2,5,11]benzoxadiazacyclote or ROS1‐positive non‐small cell tradecine‐3‐carbonitrile n/a 6/23/2015 lung cancer Pfizer, Inc. 5 (1R,3R,4R,5S)‐3‐O‐[2‐O‐ Treatment of vaso‐occlusive benzoyl‐3‐O‐(sodium(2S)‐3‐ crisis in patients with sickle cell cyclohexyl‐propanoate‐ n/a 2/17/2009 disease. Pfizer, Inc. 6 (1S)‐1‐(9‐deazahypoxanthin‐9‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ Treatment of acute Mundipharma ribitol‐hydrochloride n/a 8/13/2004 lymphoblastic leukemia Research Limited Page 1 of 359 Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 7 Treatment of chronic lymphocytic leukemia and related leukemias to include (1S)‐1‐(9‐deazahypoxanthin‐9‐ prolymphocytic leukemia, adult T‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ cell leukemia, and hairy cell Mundipharma ribitol‐hydrochloride n/a 8/10/2004 leukemia Research Ltd.