10.21608/Zumj.2021.50403.2020 962 | Page ORIGINAL ARTICLE CAN
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atrial Fibrillation and Splenic Infarction Presenting with Unexplained Fever and Persistent Abdominal Pain - a Case Report and Review of the Literature
Case ReportSplenic Infarction Presenting with Unexplained Fever and Persistent Acta Abdominal Cardiol SinPain 2012;28:157-160 Atrial Fibrillation and Splenic Infarction Presenting with Unexplained Fever and Persistent Abdominal Pain - A Case Report and Review of the Literature Cheng-Chun Wei1 and Chiung-Zuan Chiu1,2 Atrial fibrillation is a common clinical problem and may be complicated with events of thromboembolism, especially in patients with valvular heart disease. Splenic infarction is a rare manifestation of the reported cases. The symptoms may vary from asymptomatic to severe peritonitis, though early diagnosis may lessen the probability of severe complications and lead to a good prognosis. We report a 79-year-old man with multiple cardioembolic risk factors who presented with fever and left upper quadrant abdominal pain. To diagnose splenic infarction is challenging for clinicians and requires substantial effort. Early resumption of the anti-coagulation component avoids complications and operation. Key Words: Atrial fibrillation · Splenic infarction · Thromboembolism event · Valvular heart disease INTRODUCTION the patient suffered from severe acute abdominal pain due to splenic infarction. Fortunately, early diagnosis Splenic infarction is a rare cause of an acute abdo- and anticoagulation therapy helped the patient to avoid men. According to a sizeable autopsy series, only 10% emergency surgery and a possible negative outcome. of splenic infarctions had been diagnosed antemortem.1-3 It can occur in a multitude of conditions, with general or local manifestations, and was often a clinical “blind CASE REPORT spot” during the process of diagnosis. However, splenic infarction must be considered in patients with hema- The patient was a 79-year-old man with degenera- tologic diseases or thromboembolic conditions. -

New Jersey Chapter American College of Physicians
NEW JERSEY CHAPTER AMERICAN COLLEGE OF PHYSICIANS ASSOCIATES ABSTRACT COMPETITION 2015 SUBMISSIONS 2015 Resident/Fellow Abstracts 1 1. ID CATEGORY NAME ADDITIONAL PROGRAM ABSTRACT AUTHORS 2. 295 Clinical Abed, Kareem Viren Vankawala MD Atlanticare Intrapulmonary Arteriovenous Malformation causing Recurrent Cerebral Emboli Vignette FACC; Qi Sun MD Regional Medical Ischemic strokes are mainly due to cardioembolic occlusion of small vessels, as well as large vessel thromboemboli. We describe a Center case of intrapulmonary A-V shunt as the etiology of an acute ischemic event. A 63 year old male with a past history of (Dominik supraventricular tachycardia and recurrent deep vein thrombosis; who has been non-compliant on Rivaroxaban, presents with Zampino) pleuritic chest pain and was found to have a right lower lobe pulmonary embolus. The deep vein thrombosis and pulmonary embolus were not significant enough to warrant ultrasound-enhanced thrombolysis by Ekosonic EndoWave Infusion Catheter System, and the patient was subsequently restarted on Rivaroxaban and discharged. The patient presented five days later with left arm tightness and was found to have multiple areas of punctuate infarction of both cerebellar hemispheres, more confluent within the right frontal lobe. Of note he was compliant at this time with Rivaroxaban. The patient was started on unfractionated heparin drip and subsequently admitted. On admission, his vital signs showed a blood pressure of 138/93, heart rate 65 bpm, and respiratory rate 16. Cardiopulmonary examination revealed regular rate and rhythm, without murmurs, rubs or gallops and his lungs were clear to auscultation. Neurologic examination revealed intact cranial nerves, preserved strength in all extremities, mild dysmetria in the left upper extremity and an NIH score of 1. -

Summary Tabulation 10-05-2021
Summary Tabulation 10-05-2021 Active Substance (High Level) COVID-19 VACCINE ASTRAZENECA (CHADOX1 NCOV-19) Reaction SOC Reaction PT Serious Non Serious Total Blood and lymphatic system disorders Anaemia 2 1 3 Autoimmune haemolytic anaemia 1 0 1 Coagulopathy 40 21 61 Coombs negative haemolytic anaemia 1 0 1 Disseminated intravascular coagulation 1 0 1 Immune thrombocytopenia 1 0 1 Leukocytosis 1 0 1 Leukopenia 1 0 1 Lymphadenopathy 5 2 7 Lymphopenia 1 0 1 Necrotic lymphadenopathy 0 1 1 Neutropenia 3 1 4 Splenic embolism 1 0 1 Splenic infarction 1 0 1 Thrombocytopenia 19 5 24 Cardiac disorders Acute cardiac event 1 0 1 Angina pectoris 2 0 2 Arrhythmia 4 1 5 Bradycardia 1 1 2 Cardiac arrest 2 0 2 Cardiac failure 2 0 2 Cardiovascular disorder 37 6 43 Myocardial depression 1 0 1 Myocardial infarction 2 0 2 Palpitations 30 3 33 Pericarditis 1 0 1 Sinus tachycardia 1 0 1 Tachycardia 25 3 28 Ear and labyrinth disorders Deafness 1 2 3 Deafness unilateral 4 1 5 Ear congestion 0 2 2 Ear discomfort 3 0 3 Ear pain 9 2 11 Hyperacusis 3 0 3 Sudden hearing loss 0 1 1 Tinnitus 4 1 5 Vertigo 21 3 24 Endocrine disorders Adrenocortical insufficiency acute 1 0 1 Goitre 1 0 1 Eye disorders Amaurosis fugax 1 1 2 Asthenopia 2 0 2 Blindness 3 1 4 Blindness unilateral 4 0 4 Conjunctival haemorrhage 1 1 2 Eye haemorrhage 1 2 3 Eye irritation 1 0 1 Eye pain 3 2 5 Eye swelling 2 0 2 Macular oedema 1 0 1 Miosis 1 0 1 Mydriasis 1 0 1 Ocular discomfort 1 0 1 Papilloedema 1 0 1 Photophobia 5 0 5 Photopsia 1 0 1 Retinal artery thrombosis 2 0 2 Retinal ischaemia 1 0 1 Retinal -

Surgical Management of Atraumatic Splenic Rupture
International Surgery Journal Walker AM et al. Int Surg J. 2016 Nov;3(4):2280-2288 http://www.ijsurgery.com pISSN 2349-3305 | eISSN 2349-2902 DOI: http://dx.doi.org/10.18203/2349-2902.isj20163613 Case Report Surgical management of atraumatic splenic rupture Alyssa M. Walker1*, Eugene F. Foley2 1Mountain Area Health Education Center Obstetrics/Gynecology Specialists, 119 Hendersonville Road, Asheville, NC 28803, United States 2University of Wisconsin Hospital and Clinics, 621 Science Dr., Madison, WI 53711, United States Received: 04 September 2016 Accepted: 04 October 2016 *Correspondence: Dr. Alyssa Walker, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Atraumatic splenic rupture (ASR) is a rare, spontaneous, and potentially life-threatening condition that occurs in the absence of trauma; yet the management of ASR has largely defaulted to the treatment algorithm related to blunt splenic trauma. Our aim is to determine if it is appropriate and safe to use the treatment algorithm for blunt splenic trauma in the management of both pathological and non-pathological ASR. We present a case of non-pathological ASR that was successfully managed without splenectomy. A comprehensive literature review on spontaneous ASR was also performed to include publications from January 1975 to February 2015. 914 total cases of ASR were identified: 70 non-pathological and 844 pathological. Overall, 86.5% of these patients received splenectomy based on the presence or absence of traditional signs of clinical instability or deterioration, as utilized in cases of traumatic splenic rupture. -

Sickle-Cell Disorder
SICKLE-CELL DISORDER RMA ID Reference List for RMA161-2 as at June 2017 Number Addae S, Adzaku F, Mohammed S, Annobil S (1990). Sickle cell disease in 46925 permanent residents of mountain and low altitudes in Saudi Arabia. Tropical and Geographical Medicine, Vol 42 pp 342-348. Al Kahtani MA, AlQahtani M, Alshebaily MM, et al (2012). Morbidity and 79578 pregnancy outcomes associated with sickle cell anemia among Saudi women. Int J Gynecol Obstet, 119(3): 224-6. Alayed N, Kezouh A, Oddy L, et al (2014). Sickle cell disease and 79576 pregnancy outcomes: population-based study on 8.8 million births. J Perinat Med, 42(4): 487-92. Al-Salem AH (2013). Massive splenic infarction in children with sickle cell 79587 anemia and the role of splenectomy. Pediatric Surgery International, 29(3): 281-5. Ashley-Koch A, Yang Q, Olney RS (2000). Sickle hemoglobin (Hb S) allele 46299 and sickle cell disease: a HuGE review. Am J Epidemiol, 151(9):839-45. Babosa SM, Farhat SC, Martins LC, et al (2015). Air pollution and children's 79704 health: sickle cell disease. Cadernos de Saude Publica, 31(2): 265-75. Ballas SK (2007). Current issues in sickle cell pain and its management. 46357 Hematology, 2007:97-105. Barbeau P, Woods KF, Ramsey LT, Litaker MS, et al (2001). Exercise in 46995 sickle cell anemia: effect on inflammatory and vasoactive mediators. Endothelium, 8(2):147-55. Basnyat B, Tabin G (2015). Altitude Illness. Harrison's Principles of Internal 80342 Medicine, 19th Edition, 476e. Baum KF, Dunn DT, Maude GH, Serjeant GR (1987). -

An Unusual Etiology of Cytopenia, Diffuse Lymphadenopathy, and Massive Splenomegaly M
Donald and Barbara Zucker School of Medicine Journal Articles Academic Works 2015 "The Great Mimicker": An Unusual Etiology of Cytopenia, Diffuse Lymphadenopathy, and Massive Splenomegaly M. Zaarour Northwell Health C. Weerasinghe Northwell Health E. Moussaly Northwell Health S. Hussein Northwell Health J. P. Atallah Hofstra Northwell School of Medicine Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Pathology Commons Recommended Citation Zaarour M, Weerasinghe C, Moussaly E, Hussein S, Atallah J. "The Great Mimicker": An Unusual Etiology of Cytopenia, Diffuse Lymphadenopathy, and Massive Splenomegaly. 2015 Jan 01; 2015():Article 683 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/683. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. For more information, please contact [email protected]. Hindawi Publishing Corporation Case Reports in Medicine Volume 2015, Article ID 637965, 6 pages http://dx.doi.org/10.1155/2015/637965 Case Report (The Great Mimicker): An Unusual Etiology of Cytopenia, Diffuse Lymphadenopathy, and Massive Splenomegaly Mazen Zaarour,1 Chanudi Weerasinghe,1 Elias Moussaly,1 Shafinaz Hussein,2 and Jean-Paul Atallah3 1 Department of Medicine, Staten Island University Hospital, North Shore-LIJ Health System, Staten Island, New York, NY 10305, USA 2Department of Pathology, Staten Island University Hospital, North Shore-LIJ Health System, Staten Island, New York, NY 10305, USA 3Division of Hematology and Oncology, Department of Medicine, Staten Island University Hospital, North Shore-LIJ Health System, StatenIsland,NewYork,NY10305,USA Correspondence should be addressed to Mazen Zaarour; [email protected] Received 11 August 2015; Accepted 4 October 2015 Academic Editor: Masahiro Kohzuki Copyright © 2015 Mazen Zaarour et al. -

Statistical Analysis Plan
Cover Page for Statistical Analysis Plan Sponsor name: Novo Nordisk A/S NCT number NCT03061214 Sponsor trial ID: NN9535-4114 Official title of study: SUSTAINTM CHINA - Efficacy and safety of semaglutide once-weekly versus sitagliptin once-daily as add-on to metformin in subjects with type 2 diabetes Document date: 22 August 2019 Semaglutide s.c (Ozempic®) Date: 22 August 2019 Novo Nordisk Trial ID: NN9535-4114 Version: 1.0 CONFIDENTIAL Clinical Trial Report Status: Final Appendix 16.1.9 16.1.9 Documentation of statistical methods List of contents Statistical analysis plan...................................................................................................................... /LQN Statistical documentation................................................................................................................... /LQN Redacted VWDWLVWLFDODQDO\VLVSODQ Includes redaction of personal identifiable information only. Statistical Analysis Plan Date: 28 May 2019 Novo Nordisk Trial ID: NN9535-4114 Version: 1.0 CONFIDENTIAL UTN:U1111-1149-0432 Status: Final EudraCT No.:NA Page: 1 of 30 Statistical Analysis Plan Trial ID: NN9535-4114 Efficacy and safety of semaglutide once-weekly versus sitagliptin once-daily as add-on to metformin in subjects with type 2 diabetes Author Biostatistics Semaglutide s.c. This confidential document is the property of Novo Nordisk. No unpublished information contained herein may be disclosed without prior written approval from Novo Nordisk. Access to this document must be restricted to relevant parties.This -

Splenic Tuberculosis – a Rare Presentation Kafil Akhtar1, Sadaf Haiyat1, Saquib Alam1, Atia Zakaur Rab2
CASE REPORT Splenic Tuberculosis – A Rare Presentation Kafil Akhtar1, Sadaf Haiyat1, Saquib Alam1, Atia Zakaur Rab2 1Department of Pathology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh, India, 2Department of General Surgery, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh, India ABSTRACT Tuberculosis (TB) is an important health problem in developing countries, mostly seen in immunocompromised patients. Splenic TB is rare and develops as the result of either dissemination of pulmonary or biliary TB, following either ingestion of contaminated food or infected sputum. In developed countries, it is seen in patients with human immunodeficiency virus, but it is associated with significant mortality and morbidity in developing countries. We report a case of splenic TB in a 23-year-old male who presented with fever and left hypochondriac mass. A computerized tomography scan of the abdomen showed splenic enlargement with many hypodense solid to cystic lesions with ill-defined boundary. Exploratory splenectomy was performed and histological examination revealed chronic granulomatous inflammation with numerous epithelioid cells, Langhans giant cells with foci of caseous necrosis consistent with TB. He responded well with four-drug antitubercular treatment. Key words: Histopathology, spleen, tuberculosis INTRODUCTION CASE REPORT plenic tuberculosis (TB) is an extremely rare form A 23-year-old man presented to the medicine clinics with of extrapulmonary TB.[1] Non-specific clinical a history of frequent fevers for the past 2 months and Spresentation, difficulties in confirming the diagnosis, left hypochondriac fullness and discomfort for 15 days. and its subsequent treatment may lead to undue delay in the There was no history of throat pain, chest pain, cough with management of the patient. -

Splenic Infarct: a Rare Presentation of a Common Pediatric Illness
Splenic Infarct: a rare presentation of a common pediatric illness Stephanie Gehle MD, Katherine Schroeder MD, and Steven Weinberg MD Department of Pediatrics, University of North Carolina, Chapel Hill, NC Introduction Figure 2. Discussion Peripheral smear ❖ Fever, new systolic murmur and recent dental ❖ Splenic infarct is a rare manifestation of underlying without RBC procedure were concerning for infective disease, particularly in pediatric patients and morphologic endocarditis, but no vegetations were seen on patients without risk factors. abnormalities TEE. She continued to fever despite 48 hours of IV or atypical antibiotics. Other infectious etiologies were ❖ Splenic infarcts are most commonly associated with leukocytes. cardioembolic, vascular, or hematologic disorders.1 considered, though CMV PCR and EBV IgM were negative. Differential Diagnosis: ❖ Normal smear, acuity of symptoms, and lack of Acute viral or parasitic infection Presentation lymphadenopathy suggested against malignancy. Infective endocarditis History of Present Illness: Acute leukemia ❖ Though splenic infarct has been reported in autoimmune disorders, this typically is in the Previously healthy 18 year old female presented for Myelodysplastic syndrome acute onset LUQ pain after 4 days of fever, chills, context of antiphospholipid antibodies, which were and NBNB emesis. Hemoglobinopathy negative. Lab findings and lack of chronicity also Figure 1. Initial imaging: Abdominal CT at presenting helped rule out autoimmune process. Review of systems revealed headache, malaise, Hemophagocytic Lymphohistiocystosis ED showed multiple low attenuation foci concerning for muscle aches, and sore throat. No history of trauma Autoimmune disorder splenic infarcts. ❖ Splenic infarct is possible with protein C and S or coagulopathy. No known COVID exposure. deficiency, but our patient's levels were normal. -
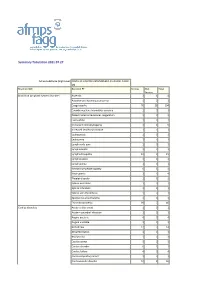
Summary Tabulation 2021.07.27
Summary Tabulation 2021.07.27 Active Substance (High Level) COVID-19 VACCINE ASTRAZENECA (CHADOX1 NCOV- 19) Reaction SOC Reaction PT Serious Non Total Serious Blood and lymphatic system disorders Anaemia 3 1 4 Autoimmune haemolytic anaemia 1 0 1 Coagulopathy 76 28 104 Coombs negative haemolytic anaemia 1 0 1 Disseminated intravascular coagulation 1 0 1 Eosinophilia 1 0 1 Immune thrombocytopenia 4 1 5 Increased tendency to bruise 1 0 1 Leukocytosis 1 0 1 Leukopenia 1 0 1 Lymph node pain 1 0 1 Lymphadenitis 1 0 1 Lymphadenopathy 12 3 15 Lymphocytosis 1 0 1 Lymphopenia 1 0 1 Necrotic lymphadenopathy 0 1 1 Neutropenia 3 1 4 Platelet disorder 1 1 2 Splenic embolism 1 0 1 Splenic infarction 1 0 1 Splenic vein thrombosis 1 0 1 Spontaneous haematoma 1 0 1 Thrombocytopenia 39 7 46 Cardiac disorders Acute cardiac event 1 0 1 Acute myocardial infarction 1 0 1 Angina pectoris 4 0 4 Angina unstable 1 0 1 Arrhythmia 12 2 14 Atrial fibrillation 1 0 1 Bradycardia 1 2 3 Cardiac arrest 3 0 3 Cardiac disorder 0 1 1 Cardiac failure 4 0 4 Cardio-respiratory arrest 1 0 1 Cardiovascular disorder 57 9 66 Cardiac disorders Coronary artery occlusion 1 0 1 Extrasystoles 1 1 2 Myocardial depression 1 0 1 Myocardial infarction 4 0 4 Myocarditis 3 0 3 Palpitations 46 13 59 Pericarditis 8 1 9 Pleuropericarditis 1 1 2 Postural orthostatic tachycardia syndrome 0 1 1 Sinus tachycardia 2 0 2 Tachyarrhythmia 1 0 1 Tachycardia 37 6 43 Congenital, familial and genetic disorders Von Willebrand's disease 1 0 1 Ear and labyrinth disorders Deafness 3 3 6 Deafness unilateral 8 -

Benign Diseases of the Spleen 7 8 Refaat B
111 2 3 10 4 5 6 Benign Diseases of the Spleen 7 8 Refaat B. Kamel 9 1011 1 2 3 4 5 6 7 8 9 2011 1 Aims ● Splenic conservation, various tech- 2 niques. 3 ● Identifying the value and functions of the ● Splenic injuries and management. 4 spleen in health and diseases. 5 ● The role of spleen in haematological dis- 6 orders (sickle cell disease, thalassaemia, Introduction 7 spherocytosis, idiopathic thrombocy- 8 topenic purpura). The spleen has always been considered a mys- 9 terious and enigmatic organ. Aristotle con- ● Haematological functions of the spleen 3011 cluded that the spleen was not essential for life. (haemopoiesis in myeloproliferative 1 As a result of this, splenectomy was undertaken disorders, red blood cell maturation, 2 lightly, without a clear understanding of subse- removal of red cell inclusions and 3 quent effects. Although Hippocrates described destruction of senescent or abnormal red 4 the anatomy of the spleen remarkably accu- cells) and immunological functions 5 rately, the exact physiology of the spleen con- (antibody production, removal of partic- 6 tinued to baffle people for more than a 1000 ulate antigens as well as clearance of 7 years after Hippocrates. The spleen was thought immune complex and phagocytosis 8 in ancient times to be the seat of emotions but (source of suppressor T cells, source 9 its real function in immunity and to remove of opsonin that promotes neutrophil 4011 time-expired blood cells and circulating phagocytosis and production of 1 microbes, has only recently been recognised. “tuftsin”). 2 3 ● Effects of splenectomy on haematologi- 4 cal and immunological functions. -

Sudden Natural Death in Infancy and Early Childhood Involving the Nervous System, a Large Number of Which Are Due to Vascular Or Infective Disorders26
STJDDEN NATI]RAL DEATII IN INFANCY & EARLY CHILDHOOD CHAPTER 4 INFE,CTIOUS CONDITIONS 't 204 ì:r INTRODUCTION ;T Sudden death is a well recognized sequel to infect¡ons with a wide variety of agents. The outcome of a particular infection depends on the age of the ¡nfant or child, the v¡rulence of the organism and the immunological status of the host. For example, certain bacteria, such as Neisseria meningidítis, are capable of producing a fulminant sept¡caemia in previously healthy children with death within hours, whereas Aspergillus sp. is generally only of concern in children who are immunodeficient. ln spite of the possibility of a lethal outcome from many 'rare' organisms in immunocompromised hosts, this chapter tends to concentrate on more usual clinical syndromes, with only occasional reference to more obscure entities. The format reflects the sequence followed in other chapters with a discussion of infections based on part¡cular organ systems or mechanisms rather than on specific classes of infectious agents. ln a review performed by the author of autopsy cases from the Adelaide Children's Hospital over the past 30 years the most common causes of rapid death were bacterial pneumonias, airway infections (including acute epiglottitis), meningitis, septicaemias, viral myocardilis and gastroenter¡t¡s. Table 4-1 lists possible microbiological causes of sudden death in childhood and Appendix l, Chapter 1 1, summarizes the types of mícrobiological specimens that can be taken in the workup of a septic case at'autopsy. 205 TABLE 4.1: TYPES OF INFECTIOUS ILLNESSES ASSOCIATED WITH SUDDEN PAEDIATR¡C DEATH 1 Cardiovascular 4.