Flood Plumes in the GBR –
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
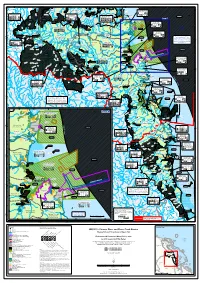
WQ1251 - Pioneer River and Plane Creek Basins Downs Mine Dam K ! R E Em E ! ! E T
! ! ! ! ! ! ! ! ! ! ! ! ! ! %2 ! ! ! ! ! 148°30'E 148°40'E 148°50'E 149°E 149°10'E 149°20'E 149°30'E ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! S ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ° k k 1 e ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! re C 2 se C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! as y ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! M y k S ! C a ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ° r ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! r Mackay City estuarine 1 %2 Proserpine River Sunset 2 a u ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! g ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! e M waters (outside port land) ! m ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Bay O k Basin ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! F C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! i ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! n Bucasia ! Upper Cattle Creek c Dalr -

Environmental Officer
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by GBRMPA eLibrary Sunfish Queensland Inc Freshwater Wetlands and Fish Importance of Freshwater Wetlands to Marine Fisheries Resources in the Great Barrier Reef Vern Veitch Bill Sawynok Report No: SQ200401 Freshwater Wetlands and Fish 1 Freshwater Wetlands and Fish Importance of Freshwater Wetlands to Marine Fisheries Resources in the Great Barrier Reef Vern Veitch1 and Bill Sawynok2 Sunfish Queensland Inc 1 Sunfish Queensland Inc 4 Stagpole Street West End Qld 4810 2 Infofish Services PO Box 9793 Frenchville Qld 4701 Published JANUARY 2005 Cover photographs: Two views of the same Gavial Creek lagoon at Rockhampton showing the extreme natural variability in wetlands depending on the weather. Information in this publication is provided as general advice only. For application to specific circumstances, professional advice should be sought. Sunfish Queensland Inc has taken all steps to ensure the information contained in this publication is accurate at the time of publication. Readers should ensure that they make the appropriate enquiries to determine whether new information is available on a particular subject matter. Report No: SQ200401 ISBN 1 876945 42 7 ¤ Great Barrier Reef Marine Park Authority and Sunfish Queensland All rights reserved. No part of this publication may be reprinted, reproduced, stored in a retrieval system or transmitted, in any form or by any means, without prior permission from the Great Barrier Reef Marine Park Authority. Freshwater Wetlands and Fish 2 Table of Contents 1. Acronyms Used in the Report .......................................................................8 2. Definition of Terms Used in the Report.........................................................9 3. -

Burnett Mary WQIP Ecologically Relevant Targets
Ecologically relevant targets for pollutant discharge from the drainage basins of the Burnett Mary Region, Great Barrier Reef TropWATER Report 14/32 Jon Brodie and Stephen Lewis 1 Ecologically relevant targets for pollutant discharge from the drainage basins of the Burnett Mary Region, Great Barrier Reef TropWATER Report 14/32 Prepared by Jon Brodie and Stephen Lewis Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER) James Cook University Townsville Phone : (07) 4781 4262 Email: [email protected] Web: www.jcu.edu.au/tropwater/ 2 Information should be cited as: Brodie J., Lewis S. (2014) Ecologically relevant targets for pollutant discharge from the drainage basins of the Burnett Mary Region, Great Barrier Reef. TropWATER Report No. 14/32, Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER), James Cook University, Townsville, 41 pp. For further information contact: Catchment to Reef Research Group/Jon Brodie and Steven Lewis Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER) James Cook University ATSIP Building Townsville, QLD 4811 [email protected] © James Cook University, 2014. Except as permitted by the Copyright Act 1968, no part of the work may in any form or by any electronic, mechanical, photocopying, recording, or any other means be reproduced, stored in a retrieval system or be broadcast or transmitted without the prior written permission of TropWATER. The information contained herein is subject to change without notice. The copyright owner shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. -

Submission DR130
To: Commissioner Dr Jane Doolan, Associate Commissioner Drew Collins Productivity Commission National Water Reform 2020 Submission by John F Kell BE (SYD), M App Sc (UNSW), MIEAust, MICE Date: 25 March 2021 Revision: 3 Summary of Contents 1.0 Introduction 2.0 Current Situation / Problem Solution 3.0 The Solution 4.0 Dam Location 5.0 Water channel design 6.0 Commonwealth of Australia Constitution Act – Section 100 7.0 Federal and State Responses 8.0 Conclusion 9.0 Acknowledgements Attachments 1 Referenced Data 2A Preliminary Design of Gravity Flow Channel Summary 2B Preliminary Design of Gravity Flow Channel Summary 3 Effectiveness of Dam Size Design Units L litres KL kilolitres ML Megalitres GL Gigalitres (Sydney Harbour ~ 500GL) GL/a Gigalitres / annum RL Relative Level - above sea level (m) m metre TEL Townsville Enterprise Limited SMEC Snowy Mountains Engineering Corporation MDBA Murray Darling Basin Authority 1.0 Introduction This submission is to present a practical solution to restore balance in the Murray Daring Basin (MDB) with a significant regular inflow of water from the Burdekin and Herbert Rivers in Queensland. My background is civil/structural engineering (BE Sydney Uni - 1973). As a fresh graduate, I worked in South Africa and UK for ~6 years, including a stint with a water consulting practice in Johannesburg, including relieving Mafeking as a site engineer on a water canal project. Attained the MICE (UK) in Manchester in 1979. In 1980 returning to Sydney, I joined Connell Wagner (now Aurecon), designing large scale industrial projects. Since 1990, I have headed a manufacturing company in the specialised field of investment casting (www.hycast.com.au) at Smithfield, NSW. -

Review of Evidence Report on Citiswich Development by Tony Loveday (Bremer Business Park Masterplan) Prepared For
A part of BMT in Energy and Environment Response Report to Floods Commission – Review of Evidence Report on Citiswich Development by Tony Loveday (Bremer Business Park Masterplan) prepared for Ipswich City Council R.B18414.005.00.doc November 2011 Response Report to Floods Commission – Review of Evidence Report on Citiswich Development by Tony Loveday (Bremer Business Park Masterplan) prepared for Ipswich City Council November 2011 Offices Brisbane Denver Mackay Melbourne Prepared For: Ipswich City Council Newcastle Perth Sydney Prepared By: BMT WBM Pty Ltd (Member of the BMT group of companies) Vancouver G:\ADMIN\B18414.G.RGS\R.B18414.005.00.DOC CONTENTS I CONTENTS Contents i 1 PURPOSE OF THE REPORT 1-1 2 HISTORY OF THE BREMER BUSINESS PARK AND CITISWICH FLOOD ASSESSMENTS 2-1 3 REVIEW OF FLOOD ASSESSMENT METHODOLOGY FOR THE PROJECT INCLUDING CUMULATIVE ASSESSMENT AND FLOOD STORAGE 3-1 4 COMPLIANCE WITH THE PLANNING SCHEME 4-1 5 LIMITATIONS OF THE REVIEW AND ASSUMPTIONS MADE BY MR LOVEDAY 5-1 6 SPECIFIC REVIEW COMMENTS ON MR LOVEDAY’S REPORT 6-1 7 CONCLUSIONS 7-1 APPENDIX A: FLOOD AND STORMWATER REPORTS – CITISWICH ESTATE A-1 APPENDIX B: BCC FILLING AND EXCAVATION CODE B-1 G:\ADMIN\B18414.G.RGS\R.B18414.005.00.DOC PURPOSE OF THE REPORT 1-1 1 PURPOSE OF THE REPORT This Report has been prepared by Neil Collins to assist Ipswich City Council with expert advice in relation to flooding in its response to a Report prepared by Mr Loveday for the Queensland Floods Commission dated 7 November 2011, in relation to the Bremer Business Park (Citiswich) Project. -

Basin-Specific Ecologically Relevant Water Quality Targets for the Great Barrier Reef
Development of basin-specific ecologically relevant water quality targets for the Great Barrier Reef Jon Brodie, Mark Baird, Jane Waterhouse, Mathieu Mongin, Jenny Skerratt, Cedric Robillot, Rachael Smith, Reinier Mann and Michael Warne TropWATER Report number 17/38 June 2017 Development of basin-specific ecologically relevant water quality targets for the Great Barrier Reef Report prepared by Jon Brodie1, Mark Baird2, Jane Waterhouse1, Mathieu Mongin2, Jenny Skerratt2, Cedric Robillot3, Rachael Smith4, Reinier Mann4 and Michael Warne4,5 2017 1James Cook University, 2CSIRO, 3eReefs, 4Department of Science, Information Technology and Innovation, 5Centre for Agroecology, Water and Resilience, Coventry University, Coventry, United Kingdom EHP16055 – Update and add to the existing 2013 Scientific Consensus Statement to incorporate the most recent science and to support the 2017 update of the Reef Water Quality Protection Plan Input and review of the development of the targets provided by John Bennett, Catherine Collier, Peter Doherty, Miles Furnas, Carol Honchin, Frederieke Kroon, Roger Shaw, Carl Mitchell and Nyssa Henry throughout the project. Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER) James Cook University Townsville Phone: (07) 4781 4262 Email: [email protected] Web: www.jcu.edu.au/tropwater/ Citation: Brodie, J., Baird, M., Waterhouse, J., Mongin, M., Skerratt, J., Robillot, C., Smith, R., Mann, R., Warne, M., 2017. Development of basin-specific ecologically relevant water quality targets for the Great Barrier -

Fisheries Guidelines for Design of Stream Crossings
Fish Habitat Guideline FHG 001 FISH PASSAGE IN STREAMS Fisheries guidelines for design of stream crossings Elizabeth Cotterell August 1998 Fisheries Group DPI ISSN 1441-1652 Agdex 486/042 FHG 001 First published August 1998 Information contained in this publication is provided as general advice only. For application to specific circumstances, professional advice should be sought. The Queensland Department of Primary Industries has taken all reasonable steps to ensure the information contained in this publication is accurate at the time of publication. Readers should ensure that they make appropriate enquiries to determine whether new information is available on the particular subject matter. © The State of Queensland, Department of Primary Industries 1998 Copyright protects this publication. Except for purposes permitted by the Copyright Act, reproduction by whatever means is prohibited without the prior written permission of the Department of Primary Industries, Queensland. Enquiries should be addressed to: Manager Publishing Services Queensland Department of Primary Industries GPO Box 46 Brisbane QLD 4001 Fisheries Guidelines for Design of Stream Crossings BACKGROUND Introduction Fish move widely in rivers and creeks throughout Queensland and Australia. Fish movement is usually associated with reproduction, feeding, escaping predators or dispersing to new habitats. This occurs between marine and freshwater habitats, and wholly within freshwater. Obstacles to this movement, such as stream crossings, can severely deplete fish populations, including recreational and commercial species such as barramundi, mullet, Mary River cod, silver perch, golden perch, sooty grunter and Australian bass. Many Queensland streams are ephemeral (they may flow only during the wet season), and therefore crossings must be designed for both flood and drought conditions. -

2012-17 Volume 2 Proserpine River Water Supply Scheme
Final Report SunWater Irrigation Price Review: 2012-17 Volume 2 Proserpine River Water Supply Scheme April 2012 Level 19, 12 Creek Street Brisbane Queensland 4000 GPO Box 2257 Brisbane Qld 4001 Telephone (07) 3222 0555 Facsimile (07) 3222 0599 [email protected] www.qca.org.au © Queensland Competition Authority 2012 The Queensland Competition Authority supports and encourages the dissemination and exchange of information. However, copyright protects this document. The Queensland Competition Authority has no objection to this material being reproduced, made available online or electronically but only if it is recognised as the owner of the copyright and this material remains unaltered. Queensland Competition Authority Table of Contents TABLE OF CONTENTS PAGE GLOSSARY III EXECUTIVE SUMMARY IV 1. PROSERPINE RIVER WATER SUPPLY SCHEME 1 1.1 Scheme Description 1 1.2 Bulk Water Infrastructure 1 1.3 Network Service Plan 2 1.4 Consultation 3 2. REGULATORY FRAMEWORK 4 2.1 Introduction 4 2.2 Draft Report 4 2.3 Submissions Received from Stakeholders on the Draft Report 6 2.4 Authority’s Response to Submissions Received on the Draft Report 6 3. PRICING FRAMEWORK 7 3.1 Tariff Structure 7 3.2 Water Use Forecasts 8 3.3 Tariff Groups 9 3.4 Kelsey Creek Water Board 10 3.5 Storage Rental Fees 10 4. RENEWALS ANNUITY 11 4.1 Background 11 4.2 SunWater’s Opening ARR Balance (1 July 2006) 12 4.3 Past Renewals Expenditure 13 4.4 Opening ARR Balance (at 1 July 2012) 16 4.5 Forecast Renewals Expenditure 17 4.6 SunWater’s Consultation with Customers 24 4.7 Allocation of Headworks Renewals Costs According to WAE Priority 25 4.8 Calculating the Renewals Annuity 29 5. -

Far North District
© The State of Queensland, 2019 © Pitney Bowes Australia Pty Ltd, 2019 © QR Limited, 2015 Based on [Dataset – Street Pro Nav] provided with the permission of Pitney Bowes Australia Pty Ltd (Current as at 12 / 19), [Dataset – Rail_Centre_Line, Oct 2015] provided with the permission of QR Limited and other state government datasets Disclaimer: While every care is taken to ensure the accuracy of this data, Pitney Bowes Australia Pty Ltd and/or the State of Queensland and/or QR Limited makes no representations or warranties about its accuracy, reliability, completeness or suitability for any particular purpose and disclaims all responsibility and all liability (including without limitation, liability in negligence) for all expenses, losses, damages (including indirect or consequential damage) and costs which you might incur as a result of the data being inaccurate or incomplete in any way and for any reason. 142°0'E 144°0'E 146°0'E 148°0'E Badu Island TORRES STRAIT ISLAND Daintree TORRES STRAIT ISLANDS ! REGIONAL COUNCIL PAPUA NEW DAINTR CAIRNS REGION Bramble Cay EE 0 4 8 12162024 p 267 Sue Islet 6 GUINEA 5 RIVE Moa Island Boigu Island 5 R Km 267 Cape Kimberley k Anchor Cay See inset for details p Saibai Island T Hawkesbury Island Dauan Island he Stephens Island ben Deliverance Island s ai Es 267 as W pla 267 TORRES SHIRE COUNCIL 266 p Wonga Beach in P na Turnagain Island G Apl de k 267 re 266 k at o Darnley Island Horn Island Little Adolphus ARAFURA iction Line Yorke Islands 9 Rd n Island Jurisd Rennel Island Dayman Point 6 n a ed 6 li d -

Queensland Floods February 2008
Report on Queensland Floods February 2008 1 2 3 4 5 6 1. Damage to boats in Airlie Beach. (John Webster) 2. High velocity flow in the Don River at the Bruce Highway Bridge in Bowen. (Burdekin Shire Council) 3. Flash flooding in Rockhampton on the 25th February. (Courier Mail Website) 4. A picture taken by the RACQ Central Queensland Rescue helicopter crew shows Mackay heavy floodwaters. 5. Hospital Bridge in Mackay during Mackay flash flood event. (ABC Website) 6. Burdekin River at Inkerman Bridge at Ayre/Home Hill. (Burdekin Shire Council) Note: 1. Data in this report has been operationally quality controlled but errors may still exist. 2. This product includes data made available to the Bureau by other agencies. Separate approval may be required to use the data for other purposes. See Appendix 1 for DNRW Usage Agreement. 3. This report is not a complete set of all data that is available. It is a representation of some of the key information. Table of Contents 1. Introduction ................................................................................................................................................... 3 Figure 1.1 Peak Flood Height Map for February 2008 - Queensland..................................................... 3 2. Meteorological Summary ............................................................................................................................. 4 2.1 Meteorological Analysis........................................................................................................................ -

Structure and Influence of Tropical River Plumes in the Great Barrier Reef: Application and Performance of an Airborne Sea Surface Salinity Mapping System
Remote Sensing of Environment 85 (2003) 204–220 www.elsevier.com/locate/rse Structure and influence of tropical river plumes in the Great Barrier Reef: application and performance of an airborne sea surface salinity mapping system D.M. Burragea,*, M.L. Heronb, J.M. Hackerc, J.L. Millerd, T.C. Stieglitza, C.R. Steinberga, A. Prytzb a Australian Institute of Marine Science, Townsville, Queensland 4810, Australia b School of Mathematical and Physical Sciences, James Cook University, Queensland 4811, Australia c Airborne Research Australia, Flinders University, Salisbury South, Adelaide, South Australia 5106, Australia d Ocean Sciences Branch, Naval Research Laboratory, Stennis Space Center, Hattiesburg, MS 39529, USA Received 29 August 2002; received in revised form 27 November 2002; accepted 2 December 2002 Abstract Input of freshwater from rivers is a critical consideration in the study and management of coral and seagrass ecosystems in tropical regions. Low salinity water can transport natural and manmade river-borne contaminants into the sea, and can directly stress marine ecosystems that are adapted to higher salinity levels. An efficient method of mapping surface salinity distribution over large ocean areas is required to address such environmental issues. We describe here an investigation of the utility of airborne remote sensing of sea surface salinity using an L-band passive microwave radiometer. The study combined aircraft overflights of the scanning low frequency microwave radiometer (SLFMR) with shipboard and in situ instrument deployments to map surface and subsurface salinity distributions, respectively, in the Great Barrier Reef Lagoon. The goals of the investigation were (a) to assess the performance of the airborne salinity mapper; (b) to use the maps and in situ data to develop an integrated description of the structure and zone of influence of a river plume under prevailing monsoon weather conditions; and (c) to determine the extent to which the sea surface salinity distribution expressed the subsurface structure. -

Surface Water Network Review Final Report
Surface Water Network Review Final Report 16 July 2018 This publication has been compiled by Operations Support - Water, Department of Natural Resources, Mines and Energy. © State of Queensland, 2018 The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. Note: Some content in this publication may have different licence terms as indicated. For more information on this licence, visit https://creativecommons.org/licenses/by/4.0/. The information contained herein is subject to change without notice. The Queensland Government shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. Interpreter statement: The Queensland Government is committed to providing accessible services to Queenslanders from all culturally and linguistically diverse backgrounds. If you have difficulty in understanding this document, you can contact us within Australia on 13QGOV (13 74 68) and we will arrange an interpreter to effectively communicate the report to you. Surface