Basin-Specific Ecologically Relevant Water Quality Targets for the Great Barrier Reef
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Queensland Public Boat Ramps
Queensland public boat ramps Ramp Location Ramp Location Atherton shire Brisbane city (cont.) Tinaroo (Church Street) Tinaroo Falls Dam Shorncliffe (Jetty Street) Cabbage Tree Creek Boat Harbour—north bank Balonne shire Shorncliffe (Sinbad Street) Cabbage Tree Creek Boat Harbour—north bank St George (Bowen Street) Jack Taylor Weir Shorncliffe (Yundah Street) Cabbage Tree Creek Boat Harbour—north bank Banana shire Wynnum (Glenora Street) Wynnum Creek—north bank Baralaba Weir Dawson River Broadsound shire Callide Dam Biloela—Calvale Road (lower ramp) Carmilla Beach (Carmilla Creek Road) Carmilla Creek—south bank, mouth of creek Callide Dam Biloela—Calvale Road (upper ramp) Clairview Beach (Colonial Drive) Clairview Beach Moura Dawson River—8 km west of Moura St Lawrence (Howards Road– Waverley Creek) Bund Creek—north bank Lake Victoria Callide Creek Bundaberg city Theodore Dawson River Bundaberg (Kirby’s Wall) Burnett River—south bank (5 km east of Bundaberg) Beaudesert shire Bundaberg (Queen Street) Burnett River—north bank (downstream) Logan River (Henderson Street– Henderson Reserve) Logan Reserve Bundaberg (Queen Street) Burnett River—north bank (upstream) Biggenden shire Burdekin shire Paradise Dam–Main Dam 500 m upstream from visitors centre Barramundi Creek (Morris Creek Road) via Hodel Road Boonah shire Cromarty Creek (Boat Ramp Road) via Giru (off the Haughton River) Groper Creek settlement Maroon Dam HG Slatter Park (Hinkson Esplanade) downstream from jetty Moogerah Dam AG Muller Park Groper Creek settlement Bowen shire (Hinkson -
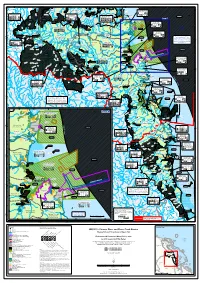
WQ1251 - Pioneer River and Plane Creek Basins Downs Mine Dam K ! R E Em E ! ! E T
! ! ! ! ! ! ! ! ! ! ! ! ! ! %2 ! ! ! ! ! 148°30'E 148°40'E 148°50'E 149°E 149°10'E 149°20'E 149°30'E ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! S ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ° k k 1 e ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! re C 2 se C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! as y ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! M y k S ! C a ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ° r ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! r Mackay City estuarine 1 %2 Proserpine River Sunset 2 a u ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! g ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! e M waters (outside port land) ! m ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Bay O k Basin ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! F C ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! i ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! n Bucasia ! Upper Cattle Creek c Dalr -

Cape York Peninsula Regional Biosecurity Plan 2016 - 2021
Cape York Peninsula Regional Biosecurity Plan 2016 - 2021 Cape York Peninsula Regional Biosecurity Plan 2016 – 2021 Page 1 Cape York Peninsula Regional Biosecurity Plan 2016 - 2021 ACKNOWLEDGMENTS This document was developed and produced by Cape York Natural Resource Management Ltd (Cape York NRM). Cape York NRM would like to acknowledge the following organisations and their officers for their contribution and support in developing the Cape York Peninsula Regional Biosecurity Plan: Cook Shire Council Northern Peninsula Area Regional Council Aurukun, Hopevale, Kowanyama Lockhart, Mapoon, Napranum, Pormpuraaw and Wujal Wujal Aboriginal Shire Councils Weipa Town Authority Rio Tinto (Alcan) Biosecurity Queensland Department of Environment and Heritage Protection Department of Natural resources and Mines Department of Agriculture and Water Resources Far North Queensland Regional Organisation of Councils Individual Cape York Peninsula Registered Native Title Body Corporates and Land Trusts Cape York Weeds and Feral Animals Incorporated Copyright 2016 Published by Cape York Natural Resource Management (Cape York NRM) Ltd. The Copyright Act 1968 permits fair dealing for study research, news reporting, criticism or review. Selected passages, tables or diagrams may be reproduced for such purposes provided acknowledgment of the source is included. Major extracts of the entire document may not be reproduced by any process without the written permission of the Chief Executive Officer, Cape York Natural Resource Management (Cape York NRM) Ltd. Please reference as: Cape York Natural Resource Management 2016, Cape York Peninsula Regional Biosecurity Plan 2016 -2021, Report prepared by the Cape York Natural Resource Management (Cape York NRM) Disclaimer: This Plan has been compiled in good faith as a basis for community and stakeholder consultation and is in draft form. -

Record of Proceedings
ISSN 1322-0330 RECORD OF PROCEEDINGS Hansard Home Page: http://www.parliament.qld.gov.au/work-of-assembly/hansard Email: [email protected] Phone (07) 3553 6344 FIRST SESSION OF THE FIFTY-SIXTH PARLIAMENT Tuesday, 26 February 2019 Subject Page ASSENT TO BILLS ............................................................................................................................................................... 311 Tabled paper: Letter, dated 21 February 2019, from His Excellency the Governor to the Speaker advising of assent to certain bills on 21 February 2019. .................................................................... 311 REPORT................................................................................................................................................................................ 311 Auditor-General ................................................................................................................................................. 311 Tabled paper: Auditor-General of Queensland: Report to Parliament No. 13: 2018-19— Health: 2017-18 results of financial audits. ....................................................................................... 311 SPEAKER’S RULING ........................................................................................................................................................... 311 Irregularities in Petition ................................................................................................................................... -

Environmental Officer
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by GBRMPA eLibrary Sunfish Queensland Inc Freshwater Wetlands and Fish Importance of Freshwater Wetlands to Marine Fisheries Resources in the Great Barrier Reef Vern Veitch Bill Sawynok Report No: SQ200401 Freshwater Wetlands and Fish 1 Freshwater Wetlands and Fish Importance of Freshwater Wetlands to Marine Fisheries Resources in the Great Barrier Reef Vern Veitch1 and Bill Sawynok2 Sunfish Queensland Inc 1 Sunfish Queensland Inc 4 Stagpole Street West End Qld 4810 2 Infofish Services PO Box 9793 Frenchville Qld 4701 Published JANUARY 2005 Cover photographs: Two views of the same Gavial Creek lagoon at Rockhampton showing the extreme natural variability in wetlands depending on the weather. Information in this publication is provided as general advice only. For application to specific circumstances, professional advice should be sought. Sunfish Queensland Inc has taken all steps to ensure the information contained in this publication is accurate at the time of publication. Readers should ensure that they make the appropriate enquiries to determine whether new information is available on a particular subject matter. Report No: SQ200401 ISBN 1 876945 42 7 ¤ Great Barrier Reef Marine Park Authority and Sunfish Queensland All rights reserved. No part of this publication may be reprinted, reproduced, stored in a retrieval system or transmitted, in any form or by any means, without prior permission from the Great Barrier Reef Marine Park Authority. Freshwater Wetlands and Fish 2 Table of Contents 1. Acronyms Used in the Report .......................................................................8 2. Definition of Terms Used in the Report.........................................................9 3. -

Christie Gallen, Kristie Thompson, Chris Paxman, Jochen Mueller
2 0 1 4 - 2 0 1 5 Christie Gallen, Kristie Thompson, Chris Paxman, Jochen Mueller Project Team - Inshore Marine Water Quality Monitoring - Christie Gallen1, Chris Paxman1, Kristie Thompson1, Elissa O’Malley1, Natalia Montero Ruiz1, Jochen Mueller1 Project Team - Assessment of Terrestrial Run-off Entering the Reef: Christie Gallen1, Kristie Thompson1, Chris Paxman1, Jochen Mueller1, Eduardo Da Silva2, Dominique O’Brien2, Dieter Tracey2, Caroline Petus2, Michelle Devlin2 1The National Research Centre for Environmental Toxicology (Entox) The University of Queensland 39 Kessels Rd Coopers Plains Qld 4108 2 The Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER), Catchment to Reef Processes Research Group, James Cook University Townsville, Qld 4811 © Copyright University of Queensland 2016 All rights are reserved and no part of this document may be reproduced, stored or copied in any form or by any means whatsoever except with the prior written permission of the University of Queensland. ISBN: 9781742721668 Report should be cited as: Gallen, C., Thompson, K., Paxman, C., Devlin, M., Mueller, J. (2016) Marine Monitoring Program. Annual Report for inshore pesticide monitoring: 2014 to 2015. Report for the Great Barrier Reef Marine Park Authority. The University of Queensland, The National Research Centre for Environmental Toxicology (Entox), Brisbane. Front cover image: View south towards the Russell River National Park and the junction of the Russell and Mulgrave Rivers over flooded sugar fields © Dieter Tracey, 2015. DISCLAIMER While reasonable efforts have been made to ensure that the contents of this document are factually correct, Entox does not make any representation or give any warranty regarding the accuracy, completeness, currency or suitability for any particular purpose of the information or statements contained in this document. -

Restricted Water Ski Areas in Queensland
Restricted Water Ski areas in Queensland Watercourse Date of Gazettal Any person operating a ship towing anyone by a line attached to the ship (including for example a person water skiing or riding on a toboggan or tube) within the waters listed below endangers marine safety. Brisbane River 20/10/2006 South Brisbane and Town Reaches of the Brisbane River between the Merivale Bridge and the Story Bridge. Burdekin River, Charters Towers 13/09/2019 All waters of The Weir on the Burdekin River, Charters Towers. Except: • commencing at a point on the waterline of the eastern bank of the Burdekin River nearest to location 19°55.279’S, 146°16.639’E, • then generally southerly along the waterline of the eastern bank to a point nearest to location 19°56.530’S, 146°17.276’E, • then westerly across Burdekin River to a point on the waterline of the western bank nearest to location 19°56.600’S, 146°17.164’E, • then generally northerly along the waterline of the western bank to a point on the waterline nearest to location 19°55.280’S, 146°16.525’E, • then easterly across the Burdekin River to the point of commencement. As shown on the map S8sp-73 prepared by Maritime Safety Queensland (MSQ) which can be found on the MSQ website at www.msq.qld.gov.au/s8sp73map and is held at MSQ’s Townsville Office. Burrum River .12/07/1996 The waters of the Burrum River within 200 metres north from the High Water mark of the southern river bank and commencing at a point 50 metres downstream of the public boat ramp off Burrum Heads Road to a point 200 metres upstream of the upstream boundary of Lions Park, Burrum Heads. -

Burnett Mary WQIP Ecologically Relevant Targets
Ecologically relevant targets for pollutant discharge from the drainage basins of the Burnett Mary Region, Great Barrier Reef TropWATER Report 14/32 Jon Brodie and Stephen Lewis 1 Ecologically relevant targets for pollutant discharge from the drainage basins of the Burnett Mary Region, Great Barrier Reef TropWATER Report 14/32 Prepared by Jon Brodie and Stephen Lewis Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER) James Cook University Townsville Phone : (07) 4781 4262 Email: [email protected] Web: www.jcu.edu.au/tropwater/ 2 Information should be cited as: Brodie J., Lewis S. (2014) Ecologically relevant targets for pollutant discharge from the drainage basins of the Burnett Mary Region, Great Barrier Reef. TropWATER Report No. 14/32, Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER), James Cook University, Townsville, 41 pp. For further information contact: Catchment to Reef Research Group/Jon Brodie and Steven Lewis Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER) James Cook University ATSIP Building Townsville, QLD 4811 [email protected] © James Cook University, 2014. Except as permitted by the Copyright Act 1968, no part of the work may in any form or by any electronic, mechanical, photocopying, recording, or any other means be reproduced, stored in a retrieval system or be broadcast or transmitted without the prior written permission of TropWATER. The information contained herein is subject to change without notice. The copyright owner shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. -

Black River Flood Study
City Wide Flood Constraints Project Townsville City Council 24-Jun-2014 Black River Flood Study Base-line Flooding Assessment J:\MMPL\60285746\8. Issued Docs\8.6 Clerical\flooding assessment\final copy\report.docx Revision A – 24-Jun-2014 Prepared for – Townsville City Council – ABN: 44 741 992 072 AECOM City Wide Flood Constraints Project Black River Flood Study Black River Flood Study Base-line Flooding Assessment Client: Townsville City Council ABN: 44 741 992 072 Prepared by AECOM Australia Pty Ltd 21 Stokes Street, PO Box 5423, Townsville QLD 4810, Australia T +61 7 4729 5500 F +61 7 4729 5599 www.aecom.com ABN 20 093 846 925 24-Jun-2014 Job No.: 60285746 AECOM in Australia and New Zealand is certified to the latest version of ISO9001, ISO14001, AS/NZS4801 and OHSAS18001. © AECOM Australia Pty Ltd (AECOM). All rights reserved. AECOM has prepared this document for the sole use of the Client and for a specific purpose, each as expressly stated in the document. No other party should rely on this document without the prior written consent of AECOM. AECOM undertakes no duty, nor accepts any responsibility, to any third party who may rely upon or use this document. This document has been prepared based on the Client’s description of its requirements and AECOM’s experience, having regard to assumptions that AECOM can reasonably be expected to make in accordance with sound professional principles. AECOM may also have relied upon information provided by the Client and other third parties to prepare this document, some of which may not have been verified. -

Lodge Fact Sheet
Baillie Lodges Finlayvale Road, Mossman QLD 4873 T 61 7 4098 1666 E [email protected] W silkyoakslodge.com.au FACT SHEET Location COOKTOWN Silky Oaks Lodge is set on 32 hectares (80 acres) of pristine rainforest above the gently flowing Mossman River, approximately 60 minutes’ drive from Cairns and GREAT BARRIER 20 minutes from Port Douglas, the gateway to the Great Barrier Reef. Also within COOKTOWN REEF ROSSVILLE easy reach are Mossman Gorge and the magnificent wilderness of Cape Tribulation. MOSSMAN CAPE TRIBULATION GORGE PORT DOUGLAS Tropical North Queensland is a remarkable region where Australia’s celebrated CAIRNS BLOOMFIELD Daintree Rainforest meets the World Heritage-listed Great Barrier Reef. At an RIVER estimated 180 million years old, the Daintree Rainforest is regarded as the oldest in the world. It is one of the most complex ecosystems on earth, containing living CAPE examples of ancient plants unique to the area as well as hundreds of species of TRIBULATION birds, animals and reptiles. The Daintree is home to the indigenous Kuku Yalanji people who have a deep connection with the land. DAINTREE RIVER CORAL SEA Climate DAINTREE Part of the Wet Tropics of Queensland World Heritage Area, the Daintree AGINCOURT DAINTREE LOW ISLES REEF climate is characterised by two distinct seasons: the dry season from April NATIONAL PARK to November offers warm days and cool nights with low humidity ranging in SILKY OAKS LODGE temperature from 19-30°C; December to March is considered the green season MOSSMAN MOSSMAN GORGE as the days and nights are hotter with more frequent restorative showers and PORT DOUGLAS temperatures between 22-32°C. -

Review of Evidence Report on Citiswich Development by Tony Loveday (Bremer Business Park Masterplan) Prepared For
A part of BMT in Energy and Environment Response Report to Floods Commission – Review of Evidence Report on Citiswich Development by Tony Loveday (Bremer Business Park Masterplan) prepared for Ipswich City Council R.B18414.005.00.doc November 2011 Response Report to Floods Commission – Review of Evidence Report on Citiswich Development by Tony Loveday (Bremer Business Park Masterplan) prepared for Ipswich City Council November 2011 Offices Brisbane Denver Mackay Melbourne Prepared For: Ipswich City Council Newcastle Perth Sydney Prepared By: BMT WBM Pty Ltd (Member of the BMT group of companies) Vancouver G:\ADMIN\B18414.G.RGS\R.B18414.005.00.DOC CONTENTS I CONTENTS Contents i 1 PURPOSE OF THE REPORT 1-1 2 HISTORY OF THE BREMER BUSINESS PARK AND CITISWICH FLOOD ASSESSMENTS 2-1 3 REVIEW OF FLOOD ASSESSMENT METHODOLOGY FOR THE PROJECT INCLUDING CUMULATIVE ASSESSMENT AND FLOOD STORAGE 3-1 4 COMPLIANCE WITH THE PLANNING SCHEME 4-1 5 LIMITATIONS OF THE REVIEW AND ASSUMPTIONS MADE BY MR LOVEDAY 5-1 6 SPECIFIC REVIEW COMMENTS ON MR LOVEDAY’S REPORT 6-1 7 CONCLUSIONS 7-1 APPENDIX A: FLOOD AND STORMWATER REPORTS – CITISWICH ESTATE A-1 APPENDIX B: BCC FILLING AND EXCAVATION CODE B-1 G:\ADMIN\B18414.G.RGS\R.B18414.005.00.DOC PURPOSE OF THE REPORT 1-1 1 PURPOSE OF THE REPORT This Report has been prepared by Neil Collins to assist Ipswich City Council with expert advice in relation to flooding in its response to a Report prepared by Mr Loveday for the Queensland Floods Commission dated 7 November 2011, in relation to the Bremer Business Park (Citiswich) Project. -

Wetlands of the Townsville Area
A Final Report to the Townsville City Council WETLANDS OF THE TOWNSVILLE AREA ACTFR Report 96/28 25 November 1996 Prepared by G. Lukacs of the Australian Centre for Tropical Freshwater Research, James Cook University of North Queensland, Townsville Q 4811 Telephone (077 814262 Facsimile (077) 815589 Wetlands of the TCC LGA: Report No.96/28 TABLE OF CONTENTS 1. INTRODUCTION .................................................................................................................................. 1 1.1 Wetlands and the Community............................................................................................................. 1 1.2 The Wetlands of the Townsville Region ............................................................................................. 1 1.3 Values and Functions of Wetlands..................................................................................................... 3 2. METHODOLOGY ................................................................................................................................. 4 2.1 Scope .................................................................................................................................................. 4 2.2 Mapping ............................................................................................................................................. 4 2.3 Classification ..................................................................................................................................... 5 2.4 Sampling............................................................................................................................................