Available Online At
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

List of 6038 Schools Selected for Establishment of Atal Tinkering
LIST OF 6038 SCHOOLS SELECTED FOR ESTABLISHMENT OF ATAL TINKERING LABS (SCHOOLS ARE KINDLY REQUESTED TO WAIT FOR FURTHER INSTRUCTIONS FROM ATAL INNOVATION MISSION, NITI AAYOG ON THEIR REGISTERED EMAIL IDs) PLEASE NOTE:- 1. LAST DATE FOR COMPLETING THE COMPLIANCE PROCESS : 31st JANUARY 2020 2. THESE SELECTED SCHOOLS MUST OPEN A NEW BANK ACCOUNT IN A PUBLIC SECTOR BANK FOR THE PURPOSE OF ATL GRANT. 3. THESE SELECTED SCHOOLS MUST NOT SHARE THEIR INFORMATION WITH ANY THIRD PARTY/ VENDOR/ AGENT/ AND MUST COMPLETE THE COMPLIANCE PROCESS ON THEIR OWN. 4. THIS LIST IS ARRANGED IN ALPHABETICAL ORDER OF STATE, DISTRICT AND FINALLY SCHOOL NAME. S.N. ATL UID CODE UDISE CODE SCHOOL NAME STATE DISTRICT 1 2760806 28222800515 ANDHRA PRADESH MODEL SCHOOL PUTLURU ANDHRA PRADESH ANANTAPUR 2 132314217 28224201013 AP MODEL SCHOOL ANDHRA PRADESH ANANTAPUR 3 574614473 28223600320 AP MODEL SCHOOL AND JUNIOR COLLEGE ANDHRA PRADESH ANANTAPUR 4 278814373 28223200124 AP MODEL SCHOOL RAPTHADU ANDHRA PRADESH ANANTAPUR 5 2995459 28222500704 AP SOCIAL WELFARE RESIDENTIAL SCHOOL JUNIOR COLLEGE FOR GIRLS KURUGUNTA ANDHRA PRADESH ANANTAPUR 6 13701194 28220601919 AVR EM HIGH SCHOOL ANDHRA PRADESH ANANTAPUR 7 15712075 28221890982 AVR EM HIGH SCHOOL ANDHRA PRADESH ANANTAPUR 8 56051196 28222301035 AVR EM HIGH SCHOOL ANDHRA PRADESH ANANTAPUR 9 385c1160 28221591153 AVR EM HIGH SCHOOL ANDHRA PRADESH ANANTAPUR 10 102112978 28220902023 GOOD SHEPHERD ENGLISH MEDIUM SCHOOL ANDHRA PRADESH ANANTAPUR 11 243715046 28220590484 K C NARAYANA E M SCHOOL ANDHRA PRADESH ANANTAPUR LIST OF 6038 SCHOOLS SELECTED FOR ESTABLISHMENT OF ATAL TINKERING LABS (SCHOOLS ARE KINDLY REQUESTED TO WAIT FOR FURTHER INSTRUCTIONS FROM ATAL INNOVATION MISSION, NITI AAYOG ON THEIR REGISTERED EMAIL IDs) PLEASE NOTE:- 1. -

Age Barred Rejected OA00004 A.Abdul Razak 15
The applications of the following candidates are REJECTED for the post of Office Assistant, for the reasons stated in the Remarks column. Reg. Name Address Date of Birth Sex Caste/ Remarks RESULT No. Community OA00001 R.Sudhakar 5/38, Arunthathiyar Street, 10/05/1979 M SC(A) Age barred Rejected Villupuram OA00004 A.Abdul Razak 64,Desurpattai Street, 15/06/1977 M BC Age barred Rejected Krishnapuram Gingee OA00006 N.Kabeer 2/46, Muslim Street, Kavarai, 02/05/1989 M BC Unsigned application Rejected Gingee taluk, Villupuram District OA00007 V.Rosalin Madhakoil Street, Kalpattu, 01/07/1983 F BC Age barred Rejected Villupuram District. OA00010 M.Gunasekaran 51, Aameena Nagar, Viratikuppam, 27/01/1983 M BC Age barred Rejected Vandimedu, Villupuram. OA00012 D.Senthil Attru Street, Karapattu, 07/04/1985 M Physically Unsigned application Rejected Eruvelpattu Post, handicappe Ulundurpet Taluk, Villupuram d District. OA00014 P.Vallabadas Madhakoil Street, Kalpattu, 05/02/1980 M BC Age barred Rejected Villupuram District. OA00022 P. Vijalakshmi No. 4/184, Lenin Nagar, Navamal 10/02/1979 F SC Age barred Rejected Marudhur, Kandamangalam Post, Villupuram Tk & Dist. OA00026 A. Bakrudeen 140/42, Agarampattai, 10/03/1977 M BC Age barred Rejected Muthuthoppu, Villupuram. OA00032 B. Arulmurugan Puthupattu Post, 16/03/1980 M SC(A) Age barred Rejected Sankarapuram Taluk Villupuram District.606402. Page 1 The applications of the following candidates are REJECTED for the post of Office Assistant, for the reasons stated in the Remarks column. Reg. Name Address Date of Birth Sex Caste/ Remarks RESULT No. Community OA00040 M.Manikandan 1/18, Periya Street, 30/05/1991 M BC Post not mentioned Rejected Sangeethamngalam Post, Villupuram District.605202. -
![292] CHENNAI, MONDAY, JULY 20, 2020 Aadi 5, Saarvari, Thiruvalluvar Aandu-2051](https://docslib.b-cdn.net/cover/7815/292-chennai-monday-july-20-2020-aadi-5-saarvari-thiruvalluvar-aandu-2051-1347815.webp)
292] CHENNAI, MONDAY, JULY 20, 2020 Aadi 5, Saarvari, Thiruvalluvar Aandu-2051
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2020 [Price: Rs. 20.80 Paise. TAMIL NADU GOVERNMENT GAZETTE EXTRAORDINARY PUBLISHED BY AUTHORITY No. 292] CHENNAI, MONDAY, JULY 20, 2020 Aadi 5, Saarvari, Thiruvalluvar Aandu-2051 Part II—Section 2 Notifi cations or Orders of interest to a Section of the public issued by Secretariat Departments. NOTIFICATIONS BY GOVERNMENT REVENUE AND DISASTER MANAGEMENT 13 Madurai 110 DEPARTMENT 14 Nagapattinam 8 COVID-19 DEMARCATION TO CONTAINMENT ZONE TO 15 Namakkal 2 CONTROL CORONA VIRUS LIST OF CONTAINMENT ZONE AS ON 17-07-2020 UNDER THE DISASTER 16 Pudukkottai 29 MANAGEMENT ACT, 2005.. 17 Ramanathapuram 17 [G.O.Ms. No. 367, Revenue and Disaster Management (D.M.II), 18 Ranipet 22 20th July 2020, Ý® 5, ꣘õK, F¼õœÀõ˜ ݇´&2051.] 19 Salem 57 No. II(2)/REVDM/443(c)/2020. 20 Sivagangai 16 The list of Containment Zones as on 17.07.2020 is notifi ed 21 Tenkasi 10 under Disaster Management Acr, 2005 for Demarcation of 22 Thanjavur 24 Containment Zone to control Corona Virus. 23 The Nilgiris 11 Abstract as on 17.07.2020 24 Theni 37 Sl. No. of District 25 Tiruvarur 55 No. Containment Zones 26 Thoothukudi 12 1 Ariyalur 7 27 Tiruchirapalli 11 2 Chengalpattu 31 28 Tirunelveli 6 3 Chennai 196 29 Tirupattur 51 4 Coimbatore 30 30 Tiruppur 115 5 Cuddalore 36 31 Tiruvallur 81 6 Dindigul 38 32 Tiruvannamalai 102 7 Erode 6 33 Vellore 7 8 Kallakurichi 35 34 Villupuram 48 9 Kancheepuram 90 35 Virudhunagar 107 10 Kanyakumari 6 Total 1423 11 Karur 1 N : Dharmapuri and Perambalur Containment completed. -

Performance Budget 2004-2005
1 PERFORMANCE BUDGET 2007-2008 BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT 1.INTRODUCTION Protection of the interests of Backward Classes, Most Backward Classes and Denotified Communities and Minorities has always been an object of prime importance of the Government of Tamil Nadu for their Socio Educational and Economic upliftment. To achieve this end the Government of Tamil Nadu is implementing multifarious welfare schemes. No State can be called as truly advanced without advancement in education. On the basis of this concept utmost importance has always been given to remove the backwardness of the people of this State, by making significant budget allocation for implementation of various educational schemes. Backward Classes/Most Backward Classes/Denotified Communities constitute the major groups among the total population of the State. Religion wise population figures in Tamil Nadu with reference to the 2001 Census are as follows:- 2 (Figures in Lakhs) % of Census year Population RELIGION 1981 1991 2001 % HINDUS 426.52 492.17 549.86 88.11 ISLAMISTS 26.91 31.06 34.7 5.56 CHRISTAINS 29.38 33.9 37.88 6.07 SIKHS 0.1 0.11 0.12 0.02 JAINS 0.63 0.73 0.81 0.13 BUDDISTS 0.05 0.06 0.065 0.01 OTHERS 0.05 0.06 0.065 0.01 NWTO STATE 0.44 0.5 0.56 0.09 Total 484.08 558.59 624.06 100 At Government level, the Secretary is heading the Department of Backward Classes, Most Backward Classes and Minorities Welfare. Different schemes and policies of the Government for the upliftment of the Backward Classes, Most Backward Classes and Denotified Communities and Minorities are implemented by the Director of Backward Classes and Minorities Welfare and Director of Most Backward Classes and Denotified Communities. -

Villupuram - District Agricultural Plan
Villupuram - District Agricultural Plan Project team Preface Foreword Executive Summary Chapter I Chapter II Chapter III Chapter IV Chapter V Chapter VI Meeting Proceedings Photos NATIONAL AGRICULTURE DEVELOPMENT PROJECT – DISTRICT AGRICULTURE PLAN PROJECT TEAM Overall Coordination : Dr. K. Palanisami, Director, CARDS and Nodal Officer (NADP) Dr. R. Venkatram, Professor and Principal Coordinator (NADP) District Level : Mrs. S. Hemalatha Coordination Assistant Professor Dept. of ARM, TNAU, Coimbatore Dr.G.Rangaraju Professor and Head Oilseed Research Station Tindivanam Dr. C. Velevan Assistant Professor Oilseed Research Station Tindivanam Mr. B. Kadarmaideen Executive Engineer (AED) Villupuram Mr. S. Rajasekaran Deputy Director of Horticulture Villupuram Mr. S. Palanivel Assistant Engineer (AED) Villupuram Mr. S. Arunagiri Assistant Engineer Public Works Department Kallakurichi Mr. T. Subramanian, Agricultural Officer Office of the Joint Director of Agriculture Villupuram Tamil Nadu Agricultural University Prof. C.RAMASAMY COIMBATORE-641 003 Vice-Chancellor TAMIL NADU INDIA. FOREWORD Date ........................... The National Development Council resolved that Agricultural Development strategies must be reoriented to meet the needs of farmers and called upon the Central and State governments to evolve a strategy to rejuvenate agriculture with a commitment to achieve four per cent annual growth in the agricultural sector during the 11th plan. The council also recommended special Additional Central Assistance Scheme named National Agriculture Development Programme (NADP) be launched. To implement this, formulation of District level action plans is the pre-requisite and thus District Agriculture Plan of various districts in Tamil Nadu has been prepared with the financial assistance of Government of India. The task of preparing the District Agriculture Plan has been given to Tamil Nadu Agricultural University by Government of Tamil Nadu. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2009-11. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2011 [Price: Rs. 33.60 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 28A] CHENNAI, WEDNESDAY, JULY 27, 2011 Aadi 11, Thiruvalluvar Aandu–2042 Part II—Section 2 (Supplement) NOTIFICATIONS BY GOVERNMENT AGRICULTURE DEPARTMENT NOTIFICATION OF CROPS, DISTRICT BLOCKS AND FIRKAS FOR KHARIF 2011UNDER NATIONAL AGRICULTURAL INSURANCE SCHEME [G.O. Rt. No. 226, Agriculture (AP1), 12th July 2001, Aani 27, Thiruvalluvar Aandu-2042.] No. II(2)/AG/347/2011. The Tamil Nadu State Level Coordination Committee on Crop Insurance have notified areas in Tamil Nadu for Paddy I (Kar/Kuruvai/Sornawari), Paddy II (Samba/Thalady/Pishanam) and Kharif (Other crops) 2011 season under National Agricultural Insurance Scheme in their Meeting held on 31.05.2011, and its subsequent minutes under reference Government letter No.11255/AP1/2011-3 dated 08.06.2011 of the Department of Agriculture, Secretariat, Fort St.George, Chennai are as below:— ABSTRACT S.No. Name of the crop. No.of Districts. No.of Blocks. No. of Firkas . I Agricultural Crops 1 Paddy-I (Kar/Kuruvai/Sornavari) 28 264 690 2 Paddy-II (Samba/Thaladi/Pishanam) 30 322 906 3 Cholam 14 92 — 4 Cumbu 10 56 — 5 Ragi 7 31 — 6 Maize 22 96 188 7 Redgram 3 31 76 8 Blackgram 7 38 95 9 Greengram 1 7 12 10 Groundnut 28 251 544 11 Gingelly 11 48 — 12 Cotton 19 86 147 13 Sunflower 16 39 .. DTP—II-2 Sup. (28A)—1 [ 1 ] 2 S. No. Name of the crop. -
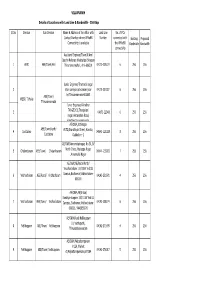
VILLUPURAM Sl.No Division Sub-Division Name & Address Of
VILLUPURAM Details of Locations with Land Line & Bandwidth - 256 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity Assistant Engineer/Town/N/Arni Opp to Nellarasi Viyabarigal Snagam 1 ARNI AEE/Town/Arni Thirumana mahal , Arni-606301 04173-225224 6 256 256 Junior Engineer/Thamarai nagar 2 Visiri samiyar ashramam(near 04175-237387 6 256 256 AEE/Town/ by)Tiruvannamalai-606603 WEST/ T.Malai Thiruvannamalai Junior Engineer/Kilnathur 3 TANGEDCO,Thenpalani 04175-223481 6 256 256 nagar,Vettavalam Road, kilnathur,Tiruvannamalai. AE/O&M,Jothinagar AEE/Town/North/ 4 Cuddalore #172,Bharathiyar Street, Kondur, 04142- 225229 8 256 256 Cuddalore Cuddalore -2 AE/O&M,Annamalainagar, No.50, IV 5 Chidambaram AEE/Town/ Chidambaram North Cross, Mariappa Nagar 04144- 239322 7 256 256 ,Annamalai Nagar AE/O&M,AE/Rural/North/ Virudhachalam 110/11KV Vrd SS 6 Vridhachalam AEE/Rural/ Vridhachalam Campus,Budhamur,Vridhachalam- 04143-231971 4 256 256 606001 AE/O&M,AE/Urban/ Kandiyankuppam 110/11KV Vrd SS 7 Vridhachalam AEE/Town/ Vridhachalam Campus, Budhamur,Vridhachalam- 04143-238274 6 256 256 606001. 9445856076 AE/O&M,Rural Nellikuppam 10, Vazhapattu, 8 Nellikuppam AEE/Town/ Nellikuppam 04142-271699 4 256 256 Thirukandeeswaram AE/O&M,Melpattampakam #12A ,Market 9 Nellikuppam AEE/Town/ Nellikuppam st,Melpattampakkam,607104 04142-276017 5 256 256 Assistant Engineer/Town I/ Villupuram, 110/11KV/SS -

Bcmbcmw E Cc 2019 20.Pdf
INDEX Sl. PAGE No SUBJET NO 1. Schemes for Backward Classes, Most Backward Classes and Denotified Communities Introduction 1 Reservation 1 Kallar Reclamation schools 2 Hostels (Boys / Girls) 4 Scholarship Schemes 9 Prizes and Awards 13 Boarding Grants 13 Rural Girls Incentive Scheme 14 Distribution of Free Bi-Cycles 14 Distribution of Free Iron Boxes 15 Distribution of Free Sewing Machines 15 Distribution of Free House site Pattas 15 Denotified Communities Welfare Board 15 Tamil Nadu Narikoravar Welfare Board 16 Tamil Nadu Vanniyakula Kshatriya Public Charitable Trusts & Endowment Board 18 Grievances redressal 18 List of Backward Classes, Backward Class Muslims, 21 Most Backward Classes and Denotified communities List of Kallar Reclamation schools 33 39 List of Hostels Sl. PAGE No SUBJET NO 2 Tamil Nadu Waqf Board Establishment 85 Functions of the board 85 Tamil Nadu Waqf Tribunal 86 Sanction of administrative grant by the Government 86 Implementation of “The Muslim Women (Protection of Rights on Divorce) Act, 1986 87 Honorarium to Kazis 87 Schemes executed by the Board with the assistance of the Government 87 Ulema Pension (Tamil Nadu) Scheme 1981 87 Corpus fund for Repair and Renovation of Mosque 88 Scheme for Major Renovation Grant for Mosque, Dargahs 88 Loan for development of Urban Waqf Properties 88 3 Tamil Nadu Backward Classes Economic Development Corporation 89 Financial Assistance Activities Eligibility Criteria 89 Mode of Application 89 Documents to be produced 89 Method of Loan sanction 90 Security for Loan Assistance 90 Scheme -

Tirukoilur Taluk, Villupuram District. W E Vulnerable Area S Firka Name:- Manalurpet Block :- Mugaiyur Taluk :- Tirukoilur District :- Villupuram
DETAILS OF AREAS VULNERABLE TO FLOOD (RURAL - FIRKA LEVEL) N TIRUKOILUR TALUK, VILLUPURAM DISTRICT. W E VULNERABLE AREA S FIRKA NAME:- MANALURPET BLOCK :- MUGAIYUR TALUK :- TIRUKOILUR DISTRICT :- VILLUPURAM 1. Details of Vulnerable Areas i a l Inundation a Distance to m Street to be a Types of Vulnerability Details of the Details- Source of Name of relief n relief n SI. No Used to relief a in the area Area Water Flood centers v Center(in Km) u Center r level(ft)/No CHENGAM TALUK i T ( TI R UV AN NA MA LAI D I ST ) o T Melandal - Low laying Area- P.U. Primary TIRUVANNAMALAI TALUK 1 Highly Vulnerable Tiruvannamalai 3 ft. School Street 500 mts Portion of Heavy Rain fall TACHCHAPPATTU Road School ( T I R U V A N N A M A L A I D I S T ) KONGANAMUR R.F 4 i a l Melandal - Heavy Rain- a Moderately P.U Primary 1 m 2 Moongilduraipattu 2 ft. Low Laying School Street 500 mts a Vulnerable School n Road n Street a v 5 u r i MELANDAL T MURUKKAMBADI o Manalurpet - P.U.Primary T 3 Low Vulnerable <2 ft. Heavy Rain School Street 500 mts ATTIYANDAL kori street School 6 2 KANGIYANUR Portion of ADUKKAM 7 R.F 2. Details of Contacts TEVADIYAKUPPAM SI. No. Name of Contact Details Contact Nos. 3 MANALURPET FRIKA 71 Sub Collector, PALLICHCHANDAL 8 10 ATTIPPAKKAM 1 Name of the Nodel Officer 9445000422 S I T H A P A T T I N A M 11 Tirukoilur JAMBAI 9 Portion of 1. -

Viluppuram District
CENSUS OF INDIA 2011 TOTAL POPULATION AND POPULATION OF SCHEDULED CASTES AND SCHEDULED TRIBES FOR VILLAGE PANCHAYATS AND PANCHAYAT UNIONS VILUPPURAM DISTRICT DIRECTORATE OF CENSUS OPERATIONS TAMILNADU ABSTRACT VILUPPURAM DISTRICT No. of Total Sl. No. Panchayat Union Total Male Total Female Total SC SC Male SC Female Total ST ST Male ST Female Village Population 1 Thirukkoyilur 52 1,27,746 65,039 62,707 42,027 21,453 20,574 398 185 213 2 Mugaiyur 63 1,96,414 99,341 97,073 59,149 30,044 29,105 1,533 766 767 3 Thiruvennainallur 49 1,35,304 68,577 66,727 47,113 23,737 23,376 277 124 153 4 Tirunavalur 44 1,32,567 67,427 65,140 44,062 22,392 21,670 245 122 123 5 Ulundurpettai 53 1,50,054 75,113 74,941 49,007 24,691 24,316 236 118 118 6 Kanai 51 1,39,738 70,672 69,066 35,458 17,836 17,622 2,465 1,219 1,246 7 Koliyanur 43 1,19,915 59,930 59,985 37,444 18,498 18,946 601 312 289 8 Kandamangalam 45 1,45,181 72,400 72,781 51,755 25,583 26,172 219 110 109 9 Vikkiravandi 50 1,22,462 61,668 60,794 38,809 19,611 19,198 1,169 602 567 10 Olakkur 52 86,700 43,373 43,327 34,541 17,342 17,199 1,839 902 937 11 Mailam 47 1,17,439 58,872 58,567 42,231 21,223 21,008 1,624 811 813 12 Marakkanam 56 1,47,713 73,944 73,769 50,833 25,399 25,434 2,103 1,030 1,073 13 Vanur 65 1,64,696 83,132 81,564 58,365 29,388 28,977 2,513 1,257 1,256 14 Gingee 60 1,39,580 70,390 69,190 31,051 15,857 15,194 3,586 1,799 1,787 15 Vallam 66 1,09,270 55,335 53,935 29,588 15,095 14,493 2,201 1,108 1,093 16 Melmalayanur 55 1,41,155 70,621 70,534 27,261 13,597 13,664 2,375 1,175 1,200 17 -

Vpm Ddo: Jao Forchief Civil Surgeon
C.P.S NEW NUMBER REPORT GOVT SERVANTS CPS PERIOD FROM 20.03.2014 TO 30.09.2014 DISTRICT VPM DDO: JAO FORCHIEF CIVIL SURGEON. MEDICAL OFFICER. GOVT HOSPITAL, VILLUPURAM -- 605 602, 1 DOJ CPS_NUMBER/SUFFIX NAME DESIGNATION DOB 7221145 / MEDL ASHIKAHAMED.R ASSISTANT SURGEON 11-05-1984 12:00:00AM16/05/2012 12:00:00AM 7221180 / MEDL ATCHAYAKUMARI.A STAFF NURSE 10-07-1986 12:00:00AM14/10/2013 12:00:00AM 7221199 / MEDL ATHIMOOLAM.S P HOSPITAL WORKER 20-07-1964 12:00:00AM03/09/2009 12:00:00AM 7221196 / MEDL BALAJI.R PHARMACIST 20-05-1969 12:00:00AM14/10/2010 12:00:00AM 7221156 / MEDL DURGA.B STAFF NURSE 05-06-1986 12:00:00AM13/09/2013 12:00:00AM 7221195 / MEDL GANAPATHY.T PHARMACIST 21-07-1975 12:00:00AM20/10/2010 12:00:00AM 7221160 / MEDL GRACY.C STAFF NURSE 30-12-1982 12:00:00AM06/12/2012 12:00:00AM 7261185 / EDN JAKULAN.T S G TEACHER 20-07-1990 12:00:00AM31/08/2012 12:00:00AM 7221194 / MEDL JAYANTHI.A PHARMACIST 29-01-1977 12:00:00AM25/09/2013 12:00:00AM 7221183 / MEDL KAVITHA.R STAFF NURSE 06-05-1985 12:00:00AM23/12/2012 12:00:00AM 7221140 / MEDL KOKILAVANI.M ASSISTANT SURGEON 15-06-1983 12:00:00AM13/02/2012 12:00:00AM 7251120 / MEDL KUPPUSAMI.P HOSPITAL WORKER 10-03-1972 12:00:00AM28/12/2010 12:00:00AM 7221198 / MEDL MUTHULAKSHMI.K RADIOGRAPHER 30-05-1987 12:00:00AM13/05/2013 12:00:00AM 7221164 / MEDL SELVI.P STAFF NURSE 24-05-1985 12:00:00AM24/11/2012 12:00:00AM 7221201 / MEDL SENTHILKUMAR.T SANITORY WORKER 03-04-1973 12:00:00AM15/10/2012 12:00:00AM 7221173 / MEDL SHARMILABEGUM.A STAFF NURSE 05-02-1986 12:00:00AM13/09/2013 12:00:00AM 7221152 -

Villupuram Sl
VILLUPURAM SL. NO. APPLICATION NO. NAME AND ADDRESS TAMILSELVAN.B 3/150-A, JANGAMINUR, 1 9856 SENGANUR, PENNAGARAM TALUK, DHARMAPURI 636810 VIJAY . C D.NO 17 G, INDRA ST, 2 9857 ANNA NAGAR, KALLAKURICHI (P.O), VILLUPURAM 606202 SAKTHIMURUGAN. R S/O RAJENDRAN THATHANOOR (VILLAGE) , 3 9858 KURUBARAHALLI (POST) , PAPPIREDDIPATTI (TALUK), DHARMAPURI 635302 CHINNA SAMY . A 777 WEST STREET, 4 9859 THOTTIYAM POST, KALLAKURCHI, VILLUPURAM 606201 KESVAN. E 18-A, KANNAGI STREET, 5 9860 KEELAPERUMBAKKAM, VILLUPURAM 605602 KRISHNAN. S G/O PERUMAL. M 31/A, STATE BANK 6 9861 COLONY BACK SIDE, BHARATHI PURAM, DHARMAPURI 636705 ANBAZHAGAN. S C/O CHINNASAMY. V 7 9862 91/76, MIDDLE STREET, PALI, VILLUPURAM 606104 TAMIL SELVAN. R 45/19E, SINGAVARAM ROAD, 8 9863 KRISHNAPURAM, GINGEE, VILLUPURAM 604202 Page 1 SARAVANAN. P S/O V.POYYATHU MARIAMMAN KOIL STREET, 9 9864 RETTANAI POST, TINDIVANAM TALUK, VILLUPURAM 604306 VENKATESAN. K 12-B, NORTH STREET, KANCHIRAPALAYAM 10 9865 COLONY & POST, KALLAKURICHI TALUK, VILLUPURAM 606207 KRISHNAMURTHI.T S/O THOPPALAN.A NO.586, SOUTH STREET, 11 9866 MEMALUR PO, TIRUKOVILUR TK, VILLUPURAM 605751 THANGAMANI.A AMMAN KOIL STREET, GOVINDHASAMY NAGAR 12 9867 (VILLAGE), VADATHORASALUR, KALLAKURICHI, VILLUPURAM 606206 SUMATHI.M D/O K.C. MADHAIYAN 7/75, AMBETHKAR STREET, 13 9868 KARIMANGALAM, PALACODE TK, DHARMAPURI 635111 ELAVARAJAN. E MARIYAMMAN KOIL STREET, 14 9869 VELIYANDHA (VILLAGE), KANJANUR (POST), VILLUPURAM 605203 RAMAKRISHNAN. M S/O MANNANGATTI. K KARUR VILLAGE, 15 9870 VAIDAPPAKKAM POST, TINDIVANAM TALUK, VILLUPURAM 604301 VENKATESHKUMAR. S S/O SUBRAMANIAN. K 2/309-A, SATHYA NAGAR, 16 9871 THADANGAM (VIA), THOKKAMPATTY POST, DHARMAPURI 636705 Page 2 ELUMALAI. K NO.139, MARIYAMMAN KOVIL STREET, 17 9872 ATHUR POST, TINDIVANAM TALUK, VILLUPURAM 604002 KESAVAN.