CORZIDE® (Nadolol and Bendroflumethiazide Tablets)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tetracaine and Oxymetazoline
PATIENT & CAREGIVER EDUCATION Tetracaine and Oxymetazoline This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. What is this drug used for? It is used before dental care to numb the area. What do I need to tell the doctor BEFORE my child takes this drug? If your child is allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell the doctor about the allergy and what signs your child had. If your child has thyroid disease, talk with the doctor. If your child has high blood pressure. If your child has methemoglobinemia. If your child is taking certain drugs used for depression like isocarboxazid, phenelzine, or tranylcypromine, or drugs used for certain other health problems like selegiline or rasagiline. If your child is taking or has recently taken any drugs for mental or mood problems like depression. There are many drugs that Tetracaine and Oxymetazoline 1/7 must not be taken with this drug. Talk with your child’s doctor if you are not sure. If your child is taking any of these drugs: Nadolol, penbutolol, pindolol, propranolol, sotalol, or timolol. If your child has used another drug in the last 24 hours that has the same drug in it. If your child is using another drug in the nose. If your child weighs less than 88 pounds (40 kilograms). This drug is not for use in children who weigh less than 88 pounds (40 kilograms). -

New Drug Evaluation Monograph Template
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 © Copyright 2012 Oregon State University. All Rights Reserved Month/Year of Review: January 2014 Date of Last Review: January 2012 PDL Classes: Beta Blockers Source Document: OSU College of Pharmacy Current Status of PDL Class: Preferred Agents: ACEBUTOLOL HCL, ATENOLOL, CARVEDILOL, LABETALOL HCL, METOPROLOL TARTRATE, NADOLOL, PROPRANOLOL HCL Non-Preferred Agents: BETAXOLOL, BISOPROLOL, METOPROLOL SUCCINATE, NEBIVOLOL (BYSTOLIC®), PENBUTOLOL (LEVABUTOL®), PINDOLOL, TIMOLOL Previous Conclusions and Recommendation: In patients with mild-moderate HF, bisoprolol, carvedilol or metoprolol succinate (ER) reduce mortality. In patients with severe HF, carvedilol or metoprolol succinate (ER) reduce mortality. In patients with recent MI, acebutolol, carvedilol, metoprolol tartrate (IR), propranolol, or timolol reduce mortality. It is important that at least one of these drugs be included in the PDL. All of the β-Blockers reviewed are effective in the treatment of hypertension, but there is no evidence of differences between β-blockers for blood pressure control, survival, or quality of life. All of the β-Blockers reviewed except carteolol reduced anginal attacks in patients in short-term studies that did not allow mortality evaluation. Because of their effectiveness in rate control for atrial fibrillation at least one of either atenolol, bisoprolol, carvedilol, metoprolol succinate (ER), nadolol, pindolol, or propranolol should be included in the PDL. The current evidence does not distinguish a difference among these beneficial β−Blockers that were tested for preventing recurrence and diminishing the severity of migraine headaches: atenolol, bisoprolol, metoprolol tartrate (IR), metoprolol succinate (ER), propranolol, propranolol LA nadolol, or timolol. -

WO 2013/061161 A2 2 May 2013 (02.05.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/061161 A2 2 May 2013 (02.05.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/337 (2006.01) A61K 31/48 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/395 (2006.01) A61K 31/51 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/4174 (2006.01) A61K 31/549 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A61K 31/428 (2006.01) A61K 31/663 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (21) International Application Number: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, PCT/IB20 12/002768 ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (22) International Filing Date: NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, 25 October 2012 (25.10.2012) RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (25) Filing Language: English ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/552,922 28 October 201 1 (28. -
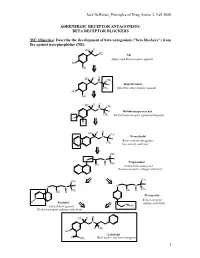
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Preventive Drug List
PREVENTIVE DRUG LIST Preventive medications are used for the prevention of conditions such as high blood pressure, high cholesterol, diabetes, asthma, osteoporosis, heart attack, stroke and prenatal nutrient deficiency. Following is a list of generic preventive medications covered at 100%, arranged by type of condition. diltiazem sotalol AF glipizide Asthma related diltiazem ER sotalol HCl glipizide ER albuterol sulfate doxazosin mesylate spironolactone glipizide/metformin HCl albuterol sulfate (nebulizer enalapril maleate spironolactone/hctz glyburide solution) enalapril maleate/hctz telmisartan glyburide micronized albuterol sulfate/ipratropium eplerenone telmisartan/amlodipine glyburide/metformin nebulizer solution eprosartan mesylate telmisartan/hctz Humalog budesonide felodipine ER terazosin HCl Humulin caffeine citrate fosinopril sodium timolol maleate Invokamet cromolyn sodium inhalation fosinopril sodium/hctz torsemide Invokamet XR solution furosemide trandolapril Invokana ipratropium bromide guanfacine HCl trandolapril/verapamil Janumet levalbuterol HCl hydralazine HCl triamterene/hctz Janumet XR levalbuterol tartrate HFA hydrochlorothiazide valsartan Januvia metaproterenol sulfate indapamide valsartan/hctz Kombiglyze XR montelukast irbesartan Vecamyl – mecamylamine HCl Lantus terbutaline sulfate irbesartan/hctz Verapamil Lantus SoloStar TheoChron isradipine Levemir theophylline anhydrous labetalol HCl Blood thinner related Levemir FlexTouch zafirlukast lisinopril aspirin/dipyridamole ER metformin HCl lisinopril/hctz cilostazol -

Medications with Fall Risk Precautions
Medications with Fall Risk Precautions Antipsychotic Drugs Factors Contributing to Fall Risk Typical Antipsychotics (First Generation) Drowsiness, dizziness, confusion, hypotension (low Chlorpromazine (Thorazine®), Fluphenazine (Prolixin®), blood pressure), orthostatic hypotension (sudden drop Haloperidol (Haldol®), Loxapine (Loxitane®), Perphenazine in blood pressure when upright), syncope (fainting), (Trilafon®), Prochlorperazine ( Compazine®), Thioridazine ataxia (gait disturbance), blurred vision, disorientation, (Mellaril®), Thiothixene (Navane®), Trifluoperazine abnormal involuntary movements of the body, slowed (Stelazine®) body movements, tremors, muscle rigidity, muscle Atypical Antipsychotics (Second Generation) spasms, seizure risk, heart rate irregularities, risk of Aripiprazole (Abilify®), Asenapine (Saphris®), Clozapine delirium (Clozaril®), Iloperidone (Fanapt®), Lurasidone (Latuda®), Olanzapine (Zyprexa®), Paliperidone (Invega®), Quetiapine (Seroquel®), Risperidone (Risperdal®), Ziprasidone (Geodon®) Anti-Anxiety Drugs Factors Contributing to Fall Risk Long-acting Benzodiazepines Drowsiness, dizziness, weakness, confusion, Chlordiazepoxide (Librium®), Clorazepate (Tranxene®), orthostatic hypotension (sudden drop in blood pressure Diazepam (Valium®), Flurazepam (Dalmane®), Clonazepam when upright), disorientation, blurred vision, (Klonopin®) unsteadiness, ataxia (gait disturbance), tremor, risk of Intermediate to Short-acting Benzodiazepines seizures (with abrupt discontinuation), blurred vision, Alprazolam (Xanax®), Lorazepam -

Cvs Caremark ® Maintenance Drug List
CVS CAREMARK® MAINTENANCE DRUG LIST EFFECTIVE AS OF 03/03/2021 Maintenance drugs are prescriptions commonly used to treat conditions that are considered chronic or long-term. These conditions usually require regular, daily use of medicines. Examples of maintenance drugs are those used to treat high blood pressure, heart disease, asthma and diabetes. Due to the large number of available medicines, this list is not all inclusive. Please note that this list does not guarantee coverage and is subject to change. Your prescription benefit plan may not cover certain products or categories, regardless of their appearance on this list. Where a generic is available, it is listed by the generic name. If no generic is available, then the brand name appears. This list represents brand products in CAPS and generic products in lower case italics. If you have questions about your prescription benefits, please log on to your account at Caremark.com or call Customer Care at the number on your ID card. Allergies pentoxifylline Depression desloratidine prasugrel bupropion levocetirizine ticlopidine citalopram ZONTIVITY desvenlafaxine Alzheimer’s Disease duloxetine donepezil Cancer escitalopram galantamine anastrozole fluoxetine memantine exemestane fluvoxamine rivastigmine letrozole mirtazapine NAMZARIC tamoxifen nefazodone toremifene paroxetine Antipsychotics sertraline trazodone Aripiprazole Contraceptives venlafaxine brexipiprazole ethinyl estradiol-desogestrel APLENZIN loxapine ethinyl estradiol-drospirenone FETZIMA olanzapine ethinyl estradiol-ethynodiol -

Controlling High Blood Pressure
CONTROLLING HIGH BLOOD PRESSURE Patients 18 to 85 years of age who had a diagnosis of hypertension (HTN) and whose blood pressure (BP) was adequately controlled during the measurement year. The blood pressure reading collected for HEDIS measure compliance must be the most recent blood pressure reading during the measurement year on or after the second diagnosis of hypertension. The blood pressure reading must be taken during an outpatient visit, non-acute inpatient encounter, or remote monitoring event (see below comments). Compliance can be captured through CPT II codes. ICD-10 I10 – Essential Hypertension OUTPATIENT CODE CPT: 99201 - 99205, 99211 - 99215, 99241 - 99245, 99341 - 99350, 99381 - 99387, 99391 - 99397, 99401 - 99404, 99411, 99412, 99429, 99455, 99456, 99483 HCPCS: G0402, G0438, G0439, G0463, T1015 NON-ACUTE INPATIENT CODES CPT: 99304 - 99310, 99315, 99316, 99318, 99324 - 99328, 99334, -99337 REMOTE BLOOD PRESSURE MONITORING CODES CPT: 93784, 93788, 93790, 99091 DESCRIPTION CPT II DESCRIPTION CPT II Diastolic 80-89 3079F Systolic > or = 140 3077F Diastolic > or = 90 3080F Systolic <140 3074F, 3075F Diastolic <80 3078F To improve HEDIS scores: • Schedule follow-up appointments and/or BP checks if BP is not controlled • Include CPT coding identified above as appropriate when submitting claims *HEDIS rules state that the last BP taken during the year on or after the date of the second diagnosis of hypertension is the only one that counts toward meeting the measure. HEDIS rules state that the organization may include BP readings from remote monitoring devices that are digitally stored and transmitted to the provider. There must be documentation in the medical record that clearly states the reading was taken by an electronic device, and results were digitally stored and transmitted to and interpreted by the provider. -

A Review of Nadolol for the Treatment of Patients with Congenital Long-QT Syndrome
A Review of Nadolol for the Treatment of Patients with Congenital Long-QT Syndrome Medicines Management Programme March 2017 Approved by Prof. Michael Barry, Clinical Lead, MMP. Date approved Version 1.0 March 2017 Version 1.0 March 2017 Contents 1. Purpose ............................................................................................................................................... 1 2. Nadolol ............................................................................................................................................... 1 2.1 Pharmacodynamics and pharmacokinetics of nadolol ............................................................... 1 2.2 Cautions and contraindications of nadolol ................................................................................. 1 2.3 Adverse-effects of nadolol ........................................................................................................... 1 2.4 Interactions of nadolol ................................................................................................................. 2 3. Background: long-QT syndrome ........................................................................................................ 2 4. Prevalence of congenital LQTS ........................................................................................................... 3 5. Treatment of congenital LQTS ........................................................................................................... 3 5.1 Beta blockers ............................................................................................................................... -

List of Formulary Drug Removals
July 2021 Formulary Drug Removals Below is a list of medicines by drug class that have been removed from your plan’s formulary. If you continue using one of the drugs listed below and identified as a Formulary Drug Removal, you may be required to pay the full cost. If you are currently using one of the formulary drug removals, ask your doctor to choose one of the generic or brand formulary options listed below. Category Formulary Drug Formulary Options Drug Class Removals Acromegaly SANDOSTATIN LAR SOMATULINE DEPOT SIGNIFOR LAR SOMAVERT Allergies dexchlorpheniramine levocetirizine Antihistamines Diphen Elixir RyClora CARBINOXAMINE TABLET 6 MG Allergies BECONASE AQ flunisolide spray, fluticasone spray, mometasone spray, DYMISTA Nasal Steroids / Combinations OMNARIS QNASL ZETONNA Anticonvulsants topiramate ext-rel capsule carbamazepine, carbamazepine ext-rel, clobazam, divalproex sodium, (generics for QUDEXY XR only) divalproex sodium ext-rel, gabapentin, lamotrigine, lamotrigine ext-rel, levetiracetam, levetiracetam ext-rel, oxcarbazepine, phenobarbital, phenytoin, phenytoin sodium extended, primidone, rufinamide, tiagabine, topiramate, valproic acid, zonisamide, FYCOMPA, OXTELLAR XR, TROKENDI XR, VIMPAT, XCOPRI BANZEL SUSPENSION clobazam, lamotrigine, rufinamide, topiramate, TROKENDI XR ONFI SABRIL vigabatrin ZONEGRAN carbamazepine, carbamazepine ext-rel, divalproex sodium, divalproex sodium ext-rel, gabapentin, lamotrigine, lamotrigine ext-rel, levetiracetam, levetiracetam ext-rel, oxcarbazepine, phenobarbital, phenytoin, phenytoin sodium -

Teva Pharmaceuticals
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF CONNECTICUT THE STATE OF CONNECTICUT; THE STATE OF ALABAMA; THE STATE OF ALASKA; Civil Action No. THE STATE OF ARIZONA; THE STATE OF COLORADO; THE STATE OF DELAWARE; THE STATE OF FLORIDA; THE STATE OF HAWAII; THE STATE OF IDAHO; May 10, 2019 THE STATE OF ILLINOIS; THE STATE OF INDIANA; THE STATE OF IOWA; COMPLAINT THE STATE OF KANSAS; THE COMMONWEALTH OF KENTUCKY; THE STATE OF LOUISIANA; THE STATE OF MAINE; THE STATE OF MARYLAND; Non-Public Version: Filed Under Seal THE COMMONWEALTH OF MASSACHUSETTS; THE STATE OF MICHIGAN; THE STATE OF MINNESOTA; THE STATE OF MISSISSIPPI; THE STATE OF MISSOURI; THE STATE OF MONTANA; THE STATE OF NEBRASKA; THE STATE OF NEVADA; THE STATE OF NEW JERSEY; THE STATE OF NEW MEXICO; THE STATE OF NEW YORK; THE STATE OF NORTH CAROLINA; THE STATE OF NORTH DAKOTA; THE STATE OF OHIO; THE STATE OF OKLAHOMA; THE STATE OF OREGON; THE COMMONWEALTH OF PENNSYLVANIA; THE COMMONWEALTH OF PUERTO RICO; THE STATE OF RHODE ISLAND; THE STATE OF SOUTH CAROLINA; THE STATE OF TENNESSEE; THE STATE OF UTAH; THE STATE OF VERMONT; THE COMMONWEALTH OF VIRGINIA; THE STATE OF WASHINGTON; THE STATE OF WEST VIRGINIA; THE STATE OF WISCONSIN; v. TEVA PHARMACEUTICALS USA, INC.; ACTAVIS HOLDCO US, INC.; ACTAVIS PHARMA, INC.; AMNEAL PHARMACEUTICALS, INC.; APOTEX CORP.; ARA APRAHAMIAN; AUROBINDO PHARMA U.S.A., INC.; DAVID BERTHOLD; BRECKENRIDGE PHARMACEUTICAL, INC.; JAMES (JIM) BROWN; MAUREEN CAVANAUGH; TRACY SULLIVAN DIVALERIO; DR. REDDY'S LABORATORIES, INC.; MARC FALKIN; GLENMARK PHARMACEUTICALS, INC., USA; JAMES (JIM) GRAUSO; KEVIN GREEN; GREENSTONE LLC; ARMANDO KELLUM; LANNETT COMPANY, INC.; LUPIN PHARMACEUTICALS, INC.; MYLAN PHARMACEUTICALS INC.; JILL NAILOR; JAMES (JIM) NESTA; PAR PHARMACEUTICAL COMPANIES, INC.; NISHA PATEL; PFIZER, INC.; KONSTANTIN OSTAFICIUK; DAVID REKENTHALER; RICHARD (RICK) ROGERSON; SANDOZ, INC.; TARO PHARMACEUTICALS USA, INC. -

LEGEND: Clinical Insights for Hypertension
LEGEND: Clinical insights for hypertension Patrick Ryan, Martijn Schuemie, Marc Suchard on behalf of the LEGEND team OHDSI Symposium 12 October 2018 Current knowledge base for hypertension Head-to-head antihypertensive drug comparisons co − amilozide hydralazine minoxidil guanfacine methyldopa chlorthalidone hydrochlorothiazide bendroflumethiazide clonidine indapamide metolazone terazosin benazepril prazosin captopril Driven primarily by ALLHAT doxazosin enalapril fosinopril Aliskiren I just 3 individual drugs lisinopril labetalol moexipril carvedilol perindopril pindolol Focus: efficacy safety quinapril penbutolol ramipril carteolol trandolapril acebutolol azilsartan propranolol candesartan Can LEGEND provide nadolol eprosartan irbesartan nebivolol 1. reliable – concordant w/ RCTs losartan metoprolol olmesartan bisoprolol telmisartan 2. rich – across “all” comparators betaxolol valsartan atenolol amlodipine felodipine spironolactone isradipine 3. relevant – inform practice epleronone nicardipine nifedipine triamterene nisoldipine amiloride lacidipine diltiazem torsemide furosemide verapamil evidence? bumetanide Trials: 40 N = 102 [1148] 33K − − LEGEND knowledge base for hypertension Head-to-head HTN drug comparisons co co − − amilozide amilozide hydralazine hydralazine minoxidil minoxidil guanfacine guanfacine methyldopa methyldopa chlorthalidone chlorthalidone hydrochlorothiazide hydrochlorothiazide bendroflumethiazide bendroflumethiazide clonidine indapamide clonidine indapamide metolazone metolazone terazosin terazosin benazepril