Adrenergic Regulation of Catecholamine Secretion from Trout (Oncorhynchus Mykiss) Chromaffin Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Emerging Evidence for a Central Epinephrine-Innervated A1- Adrenergic System That Regulates Behavioral Activation and Is Impaired in Depression
Neuropsychopharmacology (2003) 28, 1387–1399 & 2003 Nature Publishing Group All rights reserved 0893-133X/03 $25.00 www.neuropsychopharmacology.org Perspective Emerging Evidence for a Central Epinephrine-Innervated a1- Adrenergic System that Regulates Behavioral Activation and is Impaired in Depression ,1 1 1 1 1 Eric A Stone* , Yan Lin , Helen Rosengarten , H Kenneth Kramer and David Quartermain 1Departments of Psychiatry and Neurology, New York University School of Medicine, New York, NY, USA Currently, most basic and clinical research on depression is focused on either central serotonergic, noradrenergic, or dopaminergic neurotransmission as affected by various etiological and predisposing factors. Recent evidence suggests that there is another system that consists of a subset of brain a1B-adrenoceptors innervated primarily by brain epinephrine (EPI) that potentially modulates the above three monoamine systems in parallel and plays a critical role in depression. The present review covers the evidence for this system and includes findings that brain a -adrenoceptors are instrumental in behavioral activation, are located near the major monoamine cell groups 1 or target areas, receive EPI as their neurotransmitter, are impaired or inhibited in depressed patients or after stress in animal models, and a are restored by a number of antidepressants. This ‘EPI- 1 system’ may therefore represent a new target system for this disorder. Neuropsychopharmacology (2003) 28, 1387–1399, advance online publication, 18 June 2003; doi:10.1038/sj.npp.1300222 Keywords: a1-adrenoceptors; epinephrine; motor activity; depression; inactivity INTRODUCTION monoaminergic systems. This new system appears to be impaired during stress and depression and thus may Depressive illness is currently believed to result from represent a new target for this disorder. -

Intrinsic Cardiac Catecholamines Help Maintain Beating Activity in Neonatal Rat Cardiomyocyte Cultures
0031-3998/04/5603-0411 PEDIATRIC RESEARCH Vol. 56, No. 3, 2004 Copyright © 2004 International Pediatric Research Foundation, Inc. Printed in U.S.A. Intrinsic Cardiac Catecholamines Help Maintain Beating Activity in Neonatal Rat Cardiomyocyte Cultures ARUNA R. NATARAJAN, QI RONG, ALEXANDER N. KATCHMAN, AND STEVEN N. EBERT Department of Pediatrics [A.R.N.], Department of Pharmacology [Q.R., A.N.K., S.N.E.], Georgetown University Medical Center, Washington, DC 20057, U.S.A. ABSTRACT In the present study, we identify intrinsic cardiac adrenergic indicate that intrinsic cardiac catecholamines help to maintain (ICA) cells in the neonatal rat heart using immunofluorescent beating rates in neonatal rat cardiomyocyte cultures via stimula- ␣  histochemical staining techniques with antibodies that specifi- tion of 1- and -adrenergic receptors. This information should cally recognize the major enzymes in the catecholamine biosyn- help to increase our understanding of the physiologic mecha- thetic pathway. ICA cells are most concentrated near the endo- nisms governing cardiovascular function in neonates. (Pediatr cardial surface of ventricular myocardium, but are also found Res 56: 411–417, 2004) sporadically throughout the heart. In primary cultures of neonatal rat cardiomyocytes, ICA cells are closely associated with clusters Abbreviations of cardiomyocytes. To investigate a potential role for intrinsi- ICA, intrinsic cardiac adrenergic cally produced catecholamines, we recorded beating rates in the TH, tyrosine hydroxylase presence and absence of the catecholamine-depleting agent re- DBH, dopamine -hydroxylase serpine or the adrenergic receptor blockers prazosin and timolol PNMT, phenylethanolamine N-methyltransferase using videomicroscopy and photodiode sensors. Our results TRITC, tetramethylrhodamine isothiocyanate show that beating rates slow significantly when endogenous DOPS, dihydroxyphenylserine catecholamines are depleted or when their action is blocked with DMEM, Dulbecco’s modified Eagle medium  ␣ either a -oran 1-adrenergic receptor antagonist. -

Tetracaine and Oxymetazoline
PATIENT & CAREGIVER EDUCATION Tetracaine and Oxymetazoline This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. What is this drug used for? It is used before dental care to numb the area. What do I need to tell the doctor BEFORE my child takes this drug? If your child is allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell the doctor about the allergy and what signs your child had. If your child has thyroid disease, talk with the doctor. If your child has high blood pressure. If your child has methemoglobinemia. If your child is taking certain drugs used for depression like isocarboxazid, phenelzine, or tranylcypromine, or drugs used for certain other health problems like selegiline or rasagiline. If your child is taking or has recently taken any drugs for mental or mood problems like depression. There are many drugs that Tetracaine and Oxymetazoline 1/7 must not be taken with this drug. Talk with your child’s doctor if you are not sure. If your child is taking any of these drugs: Nadolol, penbutolol, pindolol, propranolol, sotalol, or timolol. If your child has used another drug in the last 24 hours that has the same drug in it. If your child is using another drug in the nose. If your child weighs less than 88 pounds (40 kilograms). This drug is not for use in children who weigh less than 88 pounds (40 kilograms). -

New Drug Evaluation Monograph Template
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 © Copyright 2012 Oregon State University. All Rights Reserved Month/Year of Review: January 2014 Date of Last Review: January 2012 PDL Classes: Beta Blockers Source Document: OSU College of Pharmacy Current Status of PDL Class: Preferred Agents: ACEBUTOLOL HCL, ATENOLOL, CARVEDILOL, LABETALOL HCL, METOPROLOL TARTRATE, NADOLOL, PROPRANOLOL HCL Non-Preferred Agents: BETAXOLOL, BISOPROLOL, METOPROLOL SUCCINATE, NEBIVOLOL (BYSTOLIC®), PENBUTOLOL (LEVABUTOL®), PINDOLOL, TIMOLOL Previous Conclusions and Recommendation: In patients with mild-moderate HF, bisoprolol, carvedilol or metoprolol succinate (ER) reduce mortality. In patients with severe HF, carvedilol or metoprolol succinate (ER) reduce mortality. In patients with recent MI, acebutolol, carvedilol, metoprolol tartrate (IR), propranolol, or timolol reduce mortality. It is important that at least one of these drugs be included in the PDL. All of the β-Blockers reviewed are effective in the treatment of hypertension, but there is no evidence of differences between β-blockers for blood pressure control, survival, or quality of life. All of the β-Blockers reviewed except carteolol reduced anginal attacks in patients in short-term studies that did not allow mortality evaluation. Because of their effectiveness in rate control for atrial fibrillation at least one of either atenolol, bisoprolol, carvedilol, metoprolol succinate (ER), nadolol, pindolol, or propranolol should be included in the PDL. The current evidence does not distinguish a difference among these beneficial β−Blockers that were tested for preventing recurrence and diminishing the severity of migraine headaches: atenolol, bisoprolol, metoprolol tartrate (IR), metoprolol succinate (ER), propranolol, propranolol LA nadolol, or timolol. -

WO 2013/061161 A2 2 May 2013 (02.05.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/061161 A2 2 May 2013 (02.05.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/337 (2006.01) A61K 31/48 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/395 (2006.01) A61K 31/51 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/4174 (2006.01) A61K 31/549 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A61K 31/428 (2006.01) A61K 31/663 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (21) International Application Number: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, PCT/IB20 12/002768 ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (22) International Filing Date: NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, 25 October 2012 (25.10.2012) RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (25) Filing Language: English ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/552,922 28 October 201 1 (28. -

Cocaine: Pharmacology, Effects, and Treatment of Abuse
Cocaine: Pharmacology, Effects, and Treatment of Abuse U. S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alcohol, Drug Abuse, and Mental Health Administration Cocaine: Pharmacology, Effects, and Treatment of Abuse Editor: John Grabowski, Ph.D. Division of Clinical Research National Institute on Drug Abuse NIDA Research Monograph 50 1984 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse 5600 Fishers Lane Rockville, Maryland 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 NIDA Research Monographs are prepared by the research divisions of the National Institute on Drug Abuse and published by its Office of Science The primary objective of the series is to provide critical reviews of research problem areas and techniques, the content of state-of-the-art conferences, and integrative research reviews. Its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisors MARTIN W. ADLER, Ph.D. SIDNEY, COHEN M.D. Temple University School of Medicine LosAngeles, California Philadelphia, Pennsylvania SYDNEY ARCHER, Ph.D. MARY L. JACOBSON Rensselaer Polytechnic Institute National Federation of Parents for Troy, New York Drug Free Youth RICHARD BELLEVILLE, Ph.D. Omaha, Nebraska NB Associates, Health Sciences Rockville, Maryland REESE T. JONES, M.D. KARST J. BESTMAN Langley Porter Neuropsychiatric Institute San Francisco, California Alcohol and Drug Problems Association of North America Washington, D.C. DENISE KANDEL, Ph.D. GILBERT J. BOVTIN, Ph.D. College of Physicians and Surgeons of Cornell University Medical College Columbia University New York, New York New York, New York JOSEPH V. -
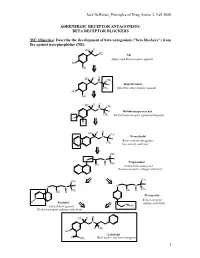
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Preventive Drug List
PREVENTIVE DRUG LIST Preventive medications are used for the prevention of conditions such as high blood pressure, high cholesterol, diabetes, asthma, osteoporosis, heart attack, stroke and prenatal nutrient deficiency. Following is a list of generic preventive medications covered at 100%, arranged by type of condition. diltiazem sotalol AF glipizide Asthma related diltiazem ER sotalol HCl glipizide ER albuterol sulfate doxazosin mesylate spironolactone glipizide/metformin HCl albuterol sulfate (nebulizer enalapril maleate spironolactone/hctz glyburide solution) enalapril maleate/hctz telmisartan glyburide micronized albuterol sulfate/ipratropium eplerenone telmisartan/amlodipine glyburide/metformin nebulizer solution eprosartan mesylate telmisartan/hctz Humalog budesonide felodipine ER terazosin HCl Humulin caffeine citrate fosinopril sodium timolol maleate Invokamet cromolyn sodium inhalation fosinopril sodium/hctz torsemide Invokamet XR solution furosemide trandolapril Invokana ipratropium bromide guanfacine HCl trandolapril/verapamil Janumet levalbuterol HCl hydralazine HCl triamterene/hctz Janumet XR levalbuterol tartrate HFA hydrochlorothiazide valsartan Januvia metaproterenol sulfate indapamide valsartan/hctz Kombiglyze XR montelukast irbesartan Vecamyl – mecamylamine HCl Lantus terbutaline sulfate irbesartan/hctz Verapamil Lantus SoloStar TheoChron isradipine Levemir theophylline anhydrous labetalol HCl Blood thinner related Levemir FlexTouch zafirlukast lisinopril aspirin/dipyridamole ER metformin HCl lisinopril/hctz cilostazol -

Neurophysiology of a Central Baroreceptor Pathway Projecting to Hypothalamic Vasopressin Neurons Jack H
LE JOURNAL CANADIEN DES SCIENCES NEUROLOGIQUES Neurophysiology of a Central Baroreceptor Pathway Projecting to Hypothalamic Vasopressin Neurons Jack H. Jhamandas and Leo P. Renaud ABSTRACT: Controversy exists as to the neural network whereby peripheral arterial baroreceptor information is transmitted to vasopressin (VP)-secreting neurons of the hypothalamic supraoptic nucleus (s.o.n.). In vivo electro physiological studies in the rat were undertaken to characterize the selective depression of VP cell activity conse quent to activation of peripheral baroreceptors. Electrical stimulation of the diagonal band of Broca (DB) in the rat evoked a similar selective inhibition of vasopressinergic neurons of the s.o.n. Local application of bicuculline, a GABA antagonist, abolished both the DB-evoked and baroreceptor-induced inhibition of VP-secreting neurons. In addition, recordings from DB neurons antidromically activated from the s.o.n. displayed an increase in firing consequent to baroreceptor activation, coinciding with the suppression of firing in s.o.n. VP neurons. These observations collectively indicate that an intrinsic GABA projection arising in the DB cell group selectively inhibits vasopressinergic neurons of the s.o.n. and that this pathway mediates peripheral arterial baroreceptor activity that influences the release of VP in the neurohypophysis. These data may be of critical importance in our understanding the etiology of those forms of experimental hypertension where abnormalities in central baroreceptor pathways have been implicated but not proven. RESUME: Neurophysiologie d'une voie centrale baroreceptrice se projetant sur les neurones vasopressinergiques de ('hypothalamus II existe une controverse concernant le reseau nerveux par lequel l'information provenant des barorecepteurs peripheriques arteriels est transmise aux neurones secretant la vasopressine (VP) au niveau du noyau hypothalamique supra-optique (n.s.o.). -

Medications with Fall Risk Precautions
Medications with Fall Risk Precautions Antipsychotic Drugs Factors Contributing to Fall Risk Typical Antipsychotics (First Generation) Drowsiness, dizziness, confusion, hypotension (low Chlorpromazine (Thorazine®), Fluphenazine (Prolixin®), blood pressure), orthostatic hypotension (sudden drop Haloperidol (Haldol®), Loxapine (Loxitane®), Perphenazine in blood pressure when upright), syncope (fainting), (Trilafon®), Prochlorperazine ( Compazine®), Thioridazine ataxia (gait disturbance), blurred vision, disorientation, (Mellaril®), Thiothixene (Navane®), Trifluoperazine abnormal involuntary movements of the body, slowed (Stelazine®) body movements, tremors, muscle rigidity, muscle Atypical Antipsychotics (Second Generation) spasms, seizure risk, heart rate irregularities, risk of Aripiprazole (Abilify®), Asenapine (Saphris®), Clozapine delirium (Clozaril®), Iloperidone (Fanapt®), Lurasidone (Latuda®), Olanzapine (Zyprexa®), Paliperidone (Invega®), Quetiapine (Seroquel®), Risperidone (Risperdal®), Ziprasidone (Geodon®) Anti-Anxiety Drugs Factors Contributing to Fall Risk Long-acting Benzodiazepines Drowsiness, dizziness, weakness, confusion, Chlordiazepoxide (Librium®), Clorazepate (Tranxene®), orthostatic hypotension (sudden drop in blood pressure Diazepam (Valium®), Flurazepam (Dalmane®), Clonazepam when upright), disorientation, blurred vision, (Klonopin®) unsteadiness, ataxia (gait disturbance), tremor, risk of Intermediate to Short-acting Benzodiazepines seizures (with abrupt discontinuation), blurred vision, Alprazolam (Xanax®), Lorazepam -

View Preprint
SIBUTRAMINE ANTINOCICEPTIVE EFFECT IN FEMALE RODENTS IS NOT DEPENDENT ON CATECHOLAMINERGIC SIGNALING Maria Luisa Azevedo de Oliveira Sales1, Karolinne Souto de Figueiredo1, Juvenia Bezerra Fontenele2, Glauce Socorro de Barros Viana1, Francisco Hélder Cavalcante Félix3 1Department of Biophysiology and Pharmacology, Faculdade de Medicina de Juazeiro do Norte. 2Department of Pharmacy, Faculdade de Farmacia, Odontologia e Enfermagem, Universidade Federal do Ceara. 3Pediatric Cancer Center, Hospital Infantil Albert Sabin Correspondence to: Francisco Hélder Cavalcante Félix, Pediatric Cancer Center, Hospital Infantil Albert Sabin, Alberto Montezuma, 350 - Vila Uniao - 60410-770 - Fortaleza - CE – Brazil, e-mail: [email protected] Running title: Sibutramine analgesia not reverted by catecholamine block Pages: 20; Abstract word count: 127; Text word count: 2443 Figures: 03; References: 15 Authorship: All authors have read and approved this manuscript. 1 PeerJ PrePrints | https://doi.org/10.7287/peerj.preprints.1544v2 | CC-BY 4.0 Open Access | rec: 30 Dec 2015, publ: 30 Dec 2015 Abstract: Sibutramine has a mechanism of action similar to that of antidepressants used as analgesics (like duloxetine). Limited data exists regarding the analgesic action of sibutramine. We tested increasing doses of p.o. sibutramine (0.1, 0.5, 1.5, 5.0 mg/kg) in the writhing test in female mice and in the plantar thermal hyperalgesia induced by carrageenan in female rats. The results showed a statistically significant (p<0.001) dose-response antinociceptive effect of sibutramine in these models, with a maximum effect comparable to the effect of a high dose of ASA (200 mg/kg) in mice and amitriptyline (10mg/kg) or indomethacin (10mg/kg) in rats. -

Cvs Caremark ® Maintenance Drug List
CVS CAREMARK® MAINTENANCE DRUG LIST EFFECTIVE AS OF 03/03/2021 Maintenance drugs are prescriptions commonly used to treat conditions that are considered chronic or long-term. These conditions usually require regular, daily use of medicines. Examples of maintenance drugs are those used to treat high blood pressure, heart disease, asthma and diabetes. Due to the large number of available medicines, this list is not all inclusive. Please note that this list does not guarantee coverage and is subject to change. Your prescription benefit plan may not cover certain products or categories, regardless of their appearance on this list. Where a generic is available, it is listed by the generic name. If no generic is available, then the brand name appears. This list represents brand products in CAPS and generic products in lower case italics. If you have questions about your prescription benefits, please log on to your account at Caremark.com or call Customer Care at the number on your ID card. Allergies pentoxifylline Depression desloratidine prasugrel bupropion levocetirizine ticlopidine citalopram ZONTIVITY desvenlafaxine Alzheimer’s Disease duloxetine donepezil Cancer escitalopram galantamine anastrozole fluoxetine memantine exemestane fluvoxamine rivastigmine letrozole mirtazapine NAMZARIC tamoxifen nefazodone toremifene paroxetine Antipsychotics sertraline trazodone Aripiprazole Contraceptives venlafaxine brexipiprazole ethinyl estradiol-desogestrel APLENZIN loxapine ethinyl estradiol-drospirenone FETZIMA olanzapine ethinyl estradiol-ethynodiol