Recycling Sewage Water for Scouring and Dyeing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2017 Walks May
Leeds Health Walks May / Jun 2017 In Association with Health for All Visit our Website www.lhwalks.wix.com/front-page MONDAY 1st May : Early May Bank Holiday NO WALK THURSDAY 1st June : no stiles 1 hill 4 miles TEMPLE NEWSAM GARDENS & ENVIRONS THURSDAY 4th May: 3.75 miles, 5 stiles, 2 hills 1 steep Leader : Pauline Ryan : Assistant Leader : Bob Marshall HUNGAR HILLS & AIRPORT Depart at 10.30am from : Home Farm / Childrens play area car Leader :John Proberts : Assistant Leader : Ian McNichol. park. From Colton road entrance to Park, at brick lodges fork Depart at 10:30 am from :Horsforth Hall Pk. From Outer Ring Rd, (A6120) turn at traffic lights (by Toby Carvery pub) into Fink Hill, left down hill to car park. LS15 0AE. first left into Hall Lane in 400yds at black GAZI'S restaurant sign, go L to bottom of road into Car Pk LS18 5JY. MONDAY 5th June : No stiles 4 miles 1 hill NEW CROFTON TO NOSTEL PRIORY CIRCULAR MONDAY 8th May : 3.5 Miles, No Stiles, slight hill Leader:Kevin O'Connor : Assistant Leader : Ian McNichol RODLEY CANAL & RIVER Depart at 10.30 am from : New Crofton Community Club, Middle Leader : John Martin : Assistant Leader : Sue Martin Lane, is at far end of Village on bend. WF4 1LD. Depart at 10:30 am from : Canal side Calverley Bridge. Turn off Ring Rd at small road down to bridge. On street parking Do not park in THURSDAY 8th June 2 hills 3 stiles 3.9 miles Railway Pub or any private car parks LS13 1PY TONG VALLEY & WOODS Leader :Henry Hamer :Assistant Leader : John Ward THURSDAY 11th May : 3.5 miles 3 stiles 1 long uphill Depart at 10: 30am from : Farnley Hall Park see details on 18th HAIGH MOOR & DOGLOITCH WOOD May LS12 5HA. -
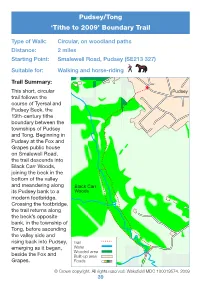
Tithe Boundary Walk – Pudsey / Tong
Pudsey/Tong ‘Tithe to 2009’ Boundary Trail Type of Walk: Circular, on woodland paths Distance: 2 miles Starting Point: Smalewell Road, Pudsey (SE213 327) Suitable for: Walking and horse-riding Trail Summary: This short, circular Pudsey trail follows the course of Tyersal and Pudsey Beck, the 19th-century tithe boundary between the townships of Pudsey and Tong. Beginning in Pudsey at the Fox and Grapes public house on Smalewell Road, the trail descends into Black Carr Woods, joining the beck in the bottom of the valley and meandering along Black Carr its Pudsey bank to a Woods modern footbridge. Crossing the footbridge, the trail returns along the beck’s opposite bank, in the township of Tong, before ascending the valley side and rising back into Pudsey, Trail emerging as it began, Water Wooded area beside the Fox and Built-up area Grapes. Roads © Crown copyright. All rights reserved. Wakefi eld MDC 100019574. 2009 39 Directions: Go around the left-hand side of the Fox and Grapes public house on Smalewell Road. Of the buildings you see here today, none was standing at the time of the Pudsey tithe map’s creation in 1847. On the site of the public house was tithe plot number 196, which was an allotment belonging to John Parker, and leased to John Sugden. Proceeding between the black and white metal gate posts to your left, onto a steeply sloping track, you begin your journey along a route frequented since at least the Victorian era. This track appears on the Pudsey tithe map as a wide vehicular access road leading from plot 197a, at the top of the hill on your left, down into Black Carr Woods. -

Historical Journey Along the River Tees and Its Tributaries
Historical Journey along the River Tees and its Tributaries Synopsis The document describes a virtual journey along the River Tees beginning at its source; the perspective is as much historical as descriptive of the current scene. Where significant tributaries join the river, they also are tracked back to their start-points. Particular attention is paid to bridges and watermills because of their intimate associations with the rivers, but nearby buildings, both religious and secular are also given attention. Some people have been specially important to developments associated with the river, and brief biographical notes are provided for them. Finally, I would stress that this is very much a personal account dealing with facets of interest to me during the 30 years or so that I spent living and working near the River Tees. Document Navigation I do not provide either a contents list, or an index, but to aid navigation through the document I give here page numbers, on which some places appear first in the text. Place Page No. Source of the River Tees 3 Middleton-in-Teesdale 5 Barnard Castle 9 River Greta confluence 15 Piercebridge 18 Darlington 20 Yarm 28 River Leven confluence 35 Stockton-on-Tees 36 Middlesbrough 37 Saltburn-by-the-Sea 46 Hartlepool 48 There is a sketch map of the river and the main tributaries in Table T1 on Page 50. The Bibliography is on Page 52. 1 River Tees and its Tributaries The River Tees flows for 135km, generally west to east from its source on the slopes of Crossfell, the highest Pennine peak, to the North Sea between Redcar and Hartlepool. -

Appendix 8G PR19 WINEP Technical Appendix
Yorkshire Water PR19 Submission | WINEP3 technical appendix 1 Appendix 8g PR19 WINEP Technical Appendix Yorkshire Water PR19 Submission | WINEP3 technical appendix 2 Introduction ................................................................................................................................................................................... ............................................. 555 1.1 Purpose of the document ............................................................................................................ 5 1.2 What is the WINEP3? ................................................................................................................... 5 WINEP: a source to sea overview ................................................................................................................................................................. ... 888 2.1 Drinking Water Protected Areas (DWPAs): uplands; water sources and habitats ..................... 8 2.2 Biodiversity ................................................................................................................................... 9 2.3 Water Framework Directive (WFD) measures and Heavily Modified Water Bodies (HMWB) .. 10 2.4 Drinking Water Protected Areas (DWPAs) Lowland water sources ........................................... 11 2.5 Wastewater impacts .................................................................................................................. 12 2.6 Customer and community benefits .......................................................................................... -
Troydale Park, Pudsey Ls28 9Lz £499,999
TROYDALE PARK, PUDSEY LS28 9LZ £499,999 BRIEFLY COMPRISING Reception Hall. Through Living Room. Morning Room / Study. Breakfast Kitchen. Dining Room / Garden Room. Guest WC. Utility Room. First Floor Staircase and Landing. Master Double Bedroom with Dressing Room and En Suite Bathroom. Two Double Bedrooms, one with En Suite Shower Room. House Bathroom. Second Floor Staircase and Study Landing. Two Further Double Bedrooms, one with Storeroom. Shower Room. Detached Double Garage. LOCATION Good access to Pudsey town centre amenities, nearby country walks in Post Hill Leeds Nature Area (LNA), private and local authority schools, local shops, parks, golf courses, supermarkets, cafes, pubs, bars and restaurants, leisure centres, swimming pool, the Aire valley with canalside walks and nature reserve, The Owlcotes Retail Centre with Asda and M&S, and commuting to Leeds and Bradford by car, bus and train. DIRECTIONS From our Pudsey office, turn left on Lidget Hill and immediately left at the traffic lights on to Lowtown. Turn right at the traffic lights on to Kent Road. At the T- junction, turn left on to Valley Road and continue down the hill as it becomes Troydale Lane. Pass the bus terminal and shortly turn left on to Troydale Park. Proceed past the shared lawns towards the head of the cul de sac where the property is located on the right and may be identified by the HomeBuyers For Sale board. Pedestrian access to the front door is through the decorative metal gate. TENURE Freehold COUNCIL TAX BAND F DISCLAIMER Details are compiled from observation and information supplied by the vendors. -
University of Huddersfield Repository
University of Huddersfield Repository Bradley, Simon Archaeology of the Voice: Exploring Oral History, Locative Media, Audio Walks, and Sound Art as Sitespecific Displacement Activities Original Citation Bradley, Simon (2016) Archaeology of the Voice: Exploring Oral History, Locative Media, Audio Walks, and Sound Art as Sitespecific Displacement Activities. Doctoral thesis, University of Huddersfield. This version is available at http://eprints.hud.ac.uk/28316/ The University Repository is a digital collection of the research output of the University, available on Open Access. Copyright and Moral Rights for the items on this site are retained by the individual author and/or other copyright owners. Users may access full items free of charge; copies of full text items generally can be reproduced, displayed or performed and given to third parties in any format or medium for personal research or study, educational or not-for-profit purposes without prior permission or charge, provided: • The authors, title and full bibliographic details is credited in any copy; • A hyperlink and/or URL is included for the original metadata page; and • The content is not changed in any way. For more information, including our policy and submission procedure, please contact the Repository Team at: [email protected]. http://eprints.hud.ac.uk/ ARCHAEOLOGY OF THE VOICE: EXPLORING ORAL HISTORY, LOCATIVE MEDIA, AUDIO WALKS, AND SOUND ART AS SITE- SPECIFIC DISPLACEMENT ACTIVITIES SIMON BRADLEY A thesis submitted to the University of Huddersfield in partial fulfillment of the requirements for the degree of Doctor of Philosophy The University of Huddersfield February 2016 Archaeology of the Voice: Exploring Oral History, Locative Media, Audio Walks, and Sound Art as Site-Specific Displacement Activities Simon Bradley Doctor of Philosophy School of Music, Humanities and Media University of Huddersfield 1 Copyright i. -

Summer Walks Leaflet 2019
Wednesday 26th June l Snow Hill in Summer Wednesday 31st July l Charlie’s Seat l 71/2 Tuesday 2nd April l Hugh ’n’ Den go l 7 miles (11.2km) l Circular l Packed lunch l miles (12km) l Circular l Packed lunch l Meet to Black Moor l 61/2 miles (10.4km) l Meet Colin Pitts at 10.30am near the canal bridge, Ings Paul Mawson at 11am at the Information Centre at the Circular l Packed lunch l Meet Paul Lane, Bradley, near Skipton. A visit to the moors and National Park Centre at Grassington. This walk follows Mawson at 10:30am at Cullingworth War farms above the village. the River Wharfe to the small village of Conistone, Memorial. An interesting walk which returning to Grassington via the moorland track. This Sunday 30th June l The Emily Bronte Stone l includes the Great Northern Railway Trail. walk includes several stiles. 6 miles (9.7km) l Circular l Packed lunch l Great views above Denholme before Meet Barbara Walker at 10:30am at Ogden Water car ascending to Black Moor and returning via park, off the A629 Denholme to Halifax Road (at Buck Park Wood. Saturday 3rd August l To Ilkley Causeway Foot). A walk which circumnavigates Nature Reserve l 2 miles (3.2km) l Thornton Moor reservoir and visits the Emily Bronte Sunday 7th April l Anne Bronte l 7 miles Circular l Snack l Meet Gillian Dale and Stone on mainly good paths and tracks. (11.2km) l Circular l Packed lunch l Meet Mary Weir at 1pm outside the Manor House, Barbara Walker at 10:30am at Haworth Church (top of Ilkley. -

Scbradleyrevisedphd.Pdf
University of Huddersfield Repository Bradley, Simon Archaeology of the Voice: Exploring Oral History, Locative Media, Audio Walks, and Sound Art as Sitespecific Displacement Activities Original Citation Bradley, Simon (2016) Archaeology of the Voice: Exploring Oral History, Locative Media, Audio Walks, and Sound Art as Sitespecific Displacement Activities. Doctoral thesis, University of Huddersfield. This version is available at http://eprints.hud.ac.uk/id/eprint/28316/ The University Repository is a digital collection of the research output of the University, available on Open Access. Copyright and Moral Rights for the items on this site are retained by the individual author and/or other copyright owners. Users may access full items free of charge; copies of full text items generally can be reproduced, displayed or performed and given to third parties in any format or medium for personal research or study, educational or not-for-profit purposes without prior permission or charge, provided: • The authors, title and full bibliographic details is credited in any copy; • A hyperlink and/or URL is included for the original metadata page; and • The content is not changed in any way. For more information, including our policy and submission procedure, please contact the Repository Team at: [email protected]. http://eprints.hud.ac.uk/ ARCHAEOLOGY OF THE VOICE: EXPLORING ORAL HISTORY, LOCATIVE MEDIA, AUDIO WALKS, AND SOUND ART AS SITE- SPECIFIC DISPLACEMENT ACTIVITIES SIMON BRADLEY A thesis submitted to the University of Huddersfield in partial fulfillment of the requirements for the degree of Doctor of Philosophy The University of Huddersfield February 2016 Archaeology of the Voice: Exploring Oral History, Locative Media, Audio Walks, and Sound Art as Site-Specific Displacement Activities Simon Bradley Doctor of Philosophy School of Music, Humanities and Media University of Huddersfield 1 Copyright i. -

Flood Risk Assessment Report
Report No. 724/01r2 FLOOD RISK ASSESSMENT REPORT AT S O U T H P A R K M I L L S P U D S E Y ON BEHALF OF A C R I V A R N L T D OCTOBER 2013 ARP ASSOCIATES CHARTERED CONSULTING ENGINEER Northwest House • 5/6 Northwest Business Park • Servia Hill • Leeds LS6 2QH Telephone: 0113 2458498 • Fax: 0113 2443864 email: [email protected] • www.arpassociates.co.uk C O N T E N T S Page 1.0 Introduction 3 2.0 Walkover Survey General 2.1 - 2.3 4 Current Use 2.4 - 2.5 4 Boundaries 2.6 5 Topography and Vegetation 2.7 5 Drainage 2.8 - 2.9 5 3.0 Environment Agency Consultation 6 4.0 Water Authority Consultation 7 5.0 Land Drainage Authority Consultation 8 6.0 Material Consideration in Respect of NPPF and TG Flood Classification 6.1 - 6.2 9 End Use 6.3 - 6.4 9 Sequential Test 6.5 - 6.6 9 Flood Sources 6.7 - 6.13 10 - 11 Climate Change 6.14 - 6.16 11 Flood Mitigation 6.17 12 Sustainable Drainage 6.18 - 6.19 12 - 13 Drainage 6.20 13 Existing Surface Water Run-off 6.21 13 Proposed Surface Water Drainage 6.22 13 - 14 Foul Drainage 6.23 14 Emergency Egress in Times of Flood 6.24 - 6.25 14 7.0 Comment 15 - 16 APPENDICES Appendix A Site Location Plan Appendix B Environment Agency Consultation Appendix C Water Authority Consultation Appendix D Land Drainage Authority Consultation Appendix E Proposed Development Layout Appendix F Indicative Surface Water Drainage Calculations 2 ARP ASSOCIATES – 724/01r2 OCTOBER 2013 1.0 INTRODUCTION 1.1 Acrivarn Ltd are considering sale of South Park Mills off Hare Lane, Pudsey with a view to redevelopment for residential properties. -

PW Integrated Template
Preliminary Design Environmental Assessment Report Volume 2: Appendices Appendix G. Noise G.1 Baseline Survey Locations Figure G-1: Site Locations C1-1 to C1-3 Figure G-1: Site Locations C2-1 to C5-1 Revision C06 Page 278 of 386 Preliminary Design Environmental Assessment Report Volume 2: Appendices Figure G-2: Site Locations C5-1 to C5-3 Figure G-1: Site Locations C6-1 to C6-3 Revision C06 Page 279 of 386 Preliminary Design Environmental Assessment Report Volume 2: Appendices G.2 Construction Activities Vs. Assessment Locations Table G-1: Day LAeq,12hr (0700 – 1900) Gantry Earthworks Gantry Excavate, Surfacing - Removal of Install Gantry Installation - Sheet Earthworks Site Installation Lay and Surfacing, Removing Address Existing Ducts and Installation - Install Earthworks Piling for - Casting Surfacing Clearance - Casting Backfill Sub-Layers Existing Structures Cabling - Piling Signals Retaining Pile Caps Pile Caps Ducts Surface and Signs Walls 77 Cottingley Drive, 0.6 -9.2 -0.2 -13.8 -18.5 -7.7 2.4 -2.9 -13.7 -18.3 -25.3 -23.9 -31.1 LS11 0JR 76 Cottingley Drive, 7.8 8.4 6.7 -2.0 -6.7 -4.4 9.3 3.7 -0.9 -5.5 -14.0 -12.5 -19.8 LS11 0JP 2 Cottingley Drive, -23.8 -19.3 -24.6 -29.9 -34.5 -32.6 -22.0 -27.7 -29.9 -34.5 -33.7 -32.2 -39.5 LS11 0JG 255 Elland Road, -19.3 -16.1 -20.3 -24.8 -29.4 -27.9 -17.7 -22.8 -26.5 -31.2 -22.8 -21.4 -28.6 LS11 8TU 277 Elland Road, -18.9 -17.8 -19.7 -24.4 -29.1 -27.7 -17.1 -22.4 -27.9 -32.6 -24.6 -23.2 -30.4 LS11 8TU 35 Noster Terrace, 12.8 -15.0 11.8 -4.0 -8.7 -4.5 14.4 2.0 -10.1 -24.8 5.6 7.0 -0.2 LS11 8QF -

Yorkshire Water Draft Determination Representation
Delivering outcomes for customers – Yorkshire Water Draft Determination Representation 1 Delivering Outcomes for Customers 3 Actions and Interventions arising from the Draft Determination 3 ODI RoRE Range 5 Individual PC and ODI Representations: 8 1. Drinking Water Quality 8 1.1 Executive summary 8 1.2 Adjustment to Performance Commitment deadband 8 2. Length of River Improved 11 2.1 Executive summary 12 2.2 Implications of Ofwat Policy Approach 12 2.3 Length of River Improved Performance Commitment and Incentive 17 3. Cost of Bad Debt 19 3.1 Executive summary 19 3.2 Performance Commitment Target 19 4. Voids Verification 23 4.1 Executive summary 24 4.2 Performance commitment definition 24 4.3 Performance Commitment Target 24 4.4 Outcome Delivery Incentive 25 5. Asset Health Incentive Collars 26 5.1 Executive summary 26 5.2 Mains Repairs and Sewer Collapses 26 5.3 Sewer Collapses 30 5.4 Unplanned Outage and Treatment Works Compliance 32 6. Leakage 33 6.1 Executive Summary 33 6.2 Performance Commitment Target 33 7. Performance Commitment Annual Profiles 36 7.1 Performance Targets 36 7.2 Integrated Catchment Management 36 7.3 Biosecurity 37 7.4 Creating Value from Waste 38 7.5 Working with Others 38 Additional Information Requests 39 8. Risk of Sewer Flooding 39 8.1 Modelling Assessment 39 9. Risk of Severe Restrictions in a Drought 40 9.1 Water Resource Management Plan Position 40 Appendix 1 42 Length of River Improved 42 Appendix 2: 44 Urban Waste Water Regulations: Delivery of schemes to meet the requirements for new Sensitive Area Designations 44 2 Delivering Outcomes for Customers Actions and Interventions arising from the Draft Determination In reviewing the draft determination for performance commitments and outcome delivery incentives, we have sought to consider the implications of the actions and interventions in relation to our whole business plan. -

River Factfiles the Aire Catchment
m Environment J Agency River factfiles The Aire catchment rivers E n v ir o n m e n t A g e n c y Information Services Unit Please return or renew this item by the due date Due Date The River Aire rises high in the Pennine Hills near Malham in the Yorkshire Dales National Park. It flows in a south east direction through limestone moorland areas passing Keighley, Bingley, Bradford and Leeds. At Castleford the river turns eastward to Goole where it meets the River Ouse. Malham Tam From Keighley to Goole the river flows through heavily populated and industrialised areas. For the last 26 kilometres upstream of Goole the river is tidal. LWinterburn Res. gargraveN. SKIPTON The Aire I River Aire KEIGHLEYSv BINGLBYesholt catchment .River Worthy KIRKSTALl SALTAIRE HAWORTH BRADFORD i TEMPLE ;new sam SELBY River Ouse FAIRBURN WEST ih a d d l e s e y River Calder, egge CARLTON :a stlefo rd • _ A^AIRMYN KNOTTINGLEY SNAITH GOOLE PONTEFRACT Total catchment population: Approximately 1.1 million Until the seventeenth century, the waste that was being dumped into Total catchment drainage area: river was used by the people of the river - industrial and factory 1,100 square kilometres Leeds for drinking water but the waste found its way there too. onset of industrialisation and the Main river catchments: At the beginning of the nineteenth Otterburn Beck, Eshton Beck, dumping of sewage waste into the century the water quality was still Broughton Beck, Eller Beck, river made this a hazardous pursuit. Eastbum Beck, Silsden Beck, River good enough to support salmon Worth, Harden Beck, Bradford The Victorians had realised that downstream of Bradford but by Beck, Oil Mill Beck, Meanwood open sewers and the disposal of 1825 the river was practically Beck, Wyke Beck, Lindyke ‘night soil* - human waste - on devoid of life.