Variation of Fish Assemblages and Species Abundances in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Ogeechee River) Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Conasauga River (below Stateline) Coosa River (River Mile Zero to Hwy 100, Floyd Co.) Coosa River <32" (Hwy 100 to Stateline, Floyd Co.) >32" Coosa River (Coosa, Etowah below Thompson-Weinman dam, Oostanaula) Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. (Crisp Co.) Holly Crk. (Murray Co.) Ichawaynochaway Crk. Kinchafoonee Crk. (above Albany) Little River (above Clarks Hill Lake) Little River (above Ga. Hwy 133, Valdosta) Mill Crk. -

Wild Species 2010 the GENERAL STATUS of SPECIES in CANADA
Wild Species 2010 THE GENERAL STATUS OF SPECIES IN CANADA Canadian Endangered Species Conservation Council National General Status Working Group This report is a product from the collaboration of all provincial and territorial governments in Canada, and of the federal government. Canadian Endangered Species Conservation Council (CESCC). 2011. Wild Species 2010: The General Status of Species in Canada. National General Status Working Group: 302 pp. Available in French under title: Espèces sauvages 2010: La situation générale des espèces au Canada. ii Abstract Wild Species 2010 is the third report of the series after 2000 and 2005. The aim of the Wild Species series is to provide an overview on which species occur in Canada, in which provinces, territories or ocean regions they occur, and what is their status. Each species assessed in this report received a rank among the following categories: Extinct (0.2), Extirpated (0.1), At Risk (1), May Be At Risk (2), Sensitive (3), Secure (4), Undetermined (5), Not Assessed (6), Exotic (7) or Accidental (8). In the 2010 report, 11 950 species were assessed. Many taxonomic groups that were first assessed in the previous Wild Species reports were reassessed, such as vascular plants, freshwater mussels, odonates, butterflies, crayfishes, amphibians, reptiles, birds and mammals. Other taxonomic groups are assessed for the first time in the Wild Species 2010 report, namely lichens, mosses, spiders, predaceous diving beetles, ground beetles (including the reassessment of tiger beetles), lady beetles, bumblebees, black flies, horse flies, mosquitoes, and some selected macromoths. The overall results of this report show that the majority of Canada’s wild species are ranked Secure. -

STURGEON HABITAT QUANTIFIED by SIDE-SCAN SONAR IMAGERY By
STURGEON HABITAT QUANTIFIED BY SIDE-SCAN SONAR IMAGERY by JOHN DAVID HOOK (Under the Direction of Nathan P. Nibbelink and Douglas L. Peterson) ABSTRACT The assessment and monitoring of freshwater habitats is essential to the successful management of imperiled fishes. Recent introduction of recreational multi-beam and side-scan sonar equipment allows rapid, low cost acquisition of bathymetric data and substrate imagery in navigable waters. However, utilization of this data is hindered by a lack of established protocols for processing and classification. I surveyed 298 km of the Ogeechee River, Georgia using low-cost recreational-grade side-scan and bathymetric sonar. I assessed classification accuracy of three approaches to working with recreational- grade sonar and quantified potential spawning grounds for Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus). I demonstrate that ecologically relevant habitat variables can be derived from low-cost sonar imagery at low levels of processing effort. INDEX WORDS: Side-scan sonar, Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus), habitat, classification accuracy STURGEON HABITAT QUANTIFIED BY SIDE-SCAN SONAR IMAGERY by JOHN DAVID HOOK B.S., Cleveland State University, 2008 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2011 © 2011 John David Hook All Rights Reserved STURGEON HABITAT QUANTIFIED BY SIDE-SCAN SONAR IMAGERY By JOHN DAVID HOOK Major Professors: Nathan Nibbelink Douglas Peterson Committee: Jeffrey Hepinstall-Cymerman Thomas Jordan Electronic Version Approved: Maureen Grasso Dean of the Graduate School The University of Georgia December 201 iv DEDICATION I would like to dedicate this to my parents, John and Joyce, whose continued support, encouragement, and enthusiasm have made all my endeavors possible, and to Emily, whose love and patience seem to know no bounds. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Guidelines for Eating Fish from Georgia Waters 2017
Guidelines For Eating Fish From Georgia Waters 2017 Georgia Department of Natural Resources 2 Martin Luther King, Jr. Drive, S.E., Suite 1252 Atlanta, Georgia 30334-9000 i ii For more information on fish consumption in Georgia, contact the Georgia Department of Natural Resources. Environmental Protection Division Watershed Protection Branch 2 Martin Luther King, Jr. Drive, S.E., Suite 1152 Atlanta, GA 30334-9000 (404) 463-1511 Wildlife Resources Division 2070 U.S. Hwy. 278, S.E. Social Circle, GA 30025 (770) 918-6406 Coastal Resources Division One Conservation Way Brunswick, Ga. 31520 (912) 264-7218 Check the DNR Web Site at: http://www.gadnr.org For this booklet: Go to Environmental Protection Division at www.gaepd.org, choose publications, then fish consumption guidelines. For the current Georgia 2015 Freshwater Sport Fishing Regulations, Click on Wild- life Resources Division. Click on Fishing. Choose Fishing Regulations. Or, go to http://www.gofishgeorgia.com For more information on Coastal Fisheries and 2015 Regulations, Click on Coastal Resources Division, or go to http://CoastalGaDNR.org For information on Household Hazardous Waste (HHW) source reduction, reuse options, proper disposal or recycling, go to Georgia Department of Community Affairs at http://www.dca.state.ga.us. Call the DNR Toll Free Tip Line at 1-800-241-4113 to report fish kills, spills, sewer over- flows, dumping or poaching (24 hours a day, seven days a week). Also, report Poaching, via e-mail using [email protected] Check USEPA and USFDA for Federal Guidance on Fish Consumption USEPA: http://www.epa.gov/ost/fishadvice USFDA: http://www.cfsan.fda.gov/seafood.1html Image Credits:Covers: Duane Raver Art Collection, courtesy of the U.S. -

The U.S. Army Corps of Engineers Regulatory Program in Georgia
The U.S. Army Corps of Engineers Regulatory Program in Georgia David Lekson, PWS Kelly Finch, PWS Richard Morgan Savannah District August, 2013 US Army Corps of Engineers BUILDING STRONG® Topics § Savannah District Regulatory Division § Regulatory Efficiencies § On the Horizon 2 BUILDING STRONG® Introduction § Joined Savannah District in Jan 2013 § 25 years as Branch Chief in the Wilmington District, NC § Certified Professional Wetland Scientist with extensive field and teaching experience across the country § Participated in regional and national initiatives (wetland delineation and assessment, Mitigation/Banking); Served 6- month detail at the Pentagon in 2012 § Evaluated phosphate/sand/rock mining, wind energy, port and military projects, water supply, landfills, utilities, transportation (highway, airport, rail), and other large-scale commercial and residential projects 3 BUILDING STRONG® Georgia § Largest state east of the Mississippi River TN NC § 59,425 Square miles § Abuts 5 states § 5 Ecoregions SC § 159 Counties AL § 70,000 Miles of waterways § 7.7 Million acres of wetlands FL 4 BUILDING STRONG® Savannah Regulatory Organization Lake Lanier Piedmont Branch Mr. Ed Johnson (678) 422-2722 Morrow Coastal Branch Ms. Kelly Finch (912) 652-5503 Savannah Albany § 35 Team members § 3 Field Offices & Savannah § 3 Branches (Piedmont, Coastal, Multipurpose Management) 5 BUILDING STRONG® Regulatory Efficiencies § General Permits ► Re-issuance of existing Regional and Programmatic General Permits ► New RGP37 for Inshore GADNR Artificial Reefs -

Streamflow Maps of Georgia's Major Rivers
GEORGIA STATE DIVISION OF CONSERVATION DEPARTMENT OF MINES, MINING AND GEOLOGY GARLAND PEYTON, Director THE GEOLOGICAL SURVEY Information Circular 21 STREAMFLOW MAPS OF GEORGIA'S MAJOR RIVERS by M. T. Thomson United States Geological Survey Prepared cooperatively by the Geological Survey, United States Department of the Interior, Washington, D. C. ATLANTA 1960 STREAMFLOW MAPS OF GEORGIA'S MAJOR RIVERS by M. T. Thomson Maps are commonly used to show the approximate rates of flow at all localities along the river systems. In addition to average flow, this collection of streamflow maps of Georgia's major rivers shows features such as low flows, flood flows, storage requirements, water power, the effects of storage reservoirs and power operations, and some comparisons of streamflows in different parts of the State. Most of the information shown on the streamflow maps was taken from "The Availability and use of Water in Georgia" by M. T. Thomson, S. M. Herrick, Eugene Brown, and others pub lished as Bulletin No. 65 in December 1956 by the Georgia Department of Mines, Mining and Geo logy. The average flows reported in that publication and sho\vn on these maps were for the years 1937-1955. That publication should be consulted for detailed information. More recent streamflow information may be obtained from the Atlanta District Office of the Surface Water Branch, Water Resources Division, U. S. Geological Survey, 805 Peachtree Street, N.E., Room 609, Atlanta 8, Georgia. In order to show the streamflows and other features clearly, the river locations are distorted slightly, their lengths are not to scale, and some features are shown by block-like patterns. -

September 24, 2018
September 24, 2018 Sent via Federal eRulemaking Portal to: http://www.regulations.gov Docket Nos. FWS-HQ-ES-2018-0006 FWS-HQ-ES-2018-0007 FWS-HQ-ES-2018-0009 Bridget Fahey Chief, Division of Conservation and Classification U.S. Fish and Wildlife Service 5275 Leesburg Pike, MS: ES Falls Church, VA 22041-3808 [email protected] Craig Aubrey Chief, Division of Environmental Review Ecological Services Program U.S. Fish and Wildlife Service 5275 Leesburg Pike, MS: ES Falls Church, VA 22041 [email protected] Samuel D. Rauch, III National Marine Fisheries Service Office of Protected Resources 1315 East-West Highway Silver Spring, MD 20910 [email protected] Re: Proposed Revisions of Endangered Species Act Regulations Dear Mr. Aubrey, Ms. Fahey, and Mr. Rauch: The Southern Environmental Law Center (“SELC”) submits the following comments in opposition to the U.S. Fish and Wildlife Service’s and National Marine Fisheries Service’s proposed revisions to the Endangered Species Act’s implementing regulations.1 We submit these comments on behalf of 57 organizations working to protect the natural resources of the 1 Revision of the Regulations for Prohibitions to Threatened Wildlife and Plants, 83 Fed. Reg. 35,174 (proposed July 25, 2018) (to be codified at 50 C.F.R. pt. 17); Revision of Regulations for Interagency Cooperation, 83 Fed. Reg. 35,178 (proposed July 25, 2018) (to be codified at 50 C.F.R. pt. 402); Revision of the Regulations for Listing Species and Designating Critical Habitat, 83 Fed. Reg. 35,193 (proposed July 25, 2018) (to be codified at 50 C.F.R. -
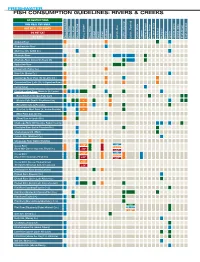
Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Lotts Crk.) Canoochee River (Lotts Crk. to Ogeechee River) Casey Canal Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Chickamauga Crk. (West) Cohulla Crk. (Whitfield Co.) Conasauga River (below Stateline) <18" Coosa River <20" 18 –32" (River Mile Zero to Hwy 100, Floyd Co.) ≥20" >32" <18" Coosa River <20" 18 –32" (Hwy 100 to Stateline, Floyd Co.) ≥20" >32" Coosa River (Coosa, Etowah below <20" Thompson-Weinman dam, Oostanaula) ≥20" Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. -

Georgia Ecological Services U.S. Fish & Wildlife Service HUC 10
Georgia Ecological Services U.S. Fish & Wildlife Service 2/9/2021 HUC 10 Watershed Report HUC 10 Watershed: 0313000514 Beaver Creek-Flint River HUC 8 Watershed: Upper Flint Counties: Crawford, Macon, Peach, Taylor, Upson Major Waterbodies (in GA): Flint River, Beaver Creek, Avery Creek, Griffin Branch, Little Vine Creek, Mathews Creek Federal Listed Species: (historic, known occurrence, or likely to occur in the watershed) E - Endangered, T - Threatened, C - Candidate, CCA - Candidate Conservation species, PE - Proposed Endangered, PT - Proposed Threatened, Pet - Petitioned, R - Rare, U - Uncommon, SC - Species of Concern. Fat Three-ridge (Amblema neislerii) US: E; GA: E Floodplain; Survey period: year round, when water temperatures are above 10° C and excluding when stage is increasing or above normal. Purple Bankclimber (Elliptoideus sloatianus) US: T; GA: T Occurrence; Critical Habitat; Survey period: year round, when water temperatures are above 10° C and excluding when stage is increasing or above normal. Shinyrayed Pocketbook (Hamiota subangulata) US: E; GA: E Occurrence; Critical Habitat; Survey period: year round, when water temperatures are above 10° C and excluding when stage is increasing or above normal. Gulf Moccasinshell (Medionidus penicillatus) US: E; GA: E Occurrence; Critical Habitat; Survey period: for larvae or aquatic adults between 1 Apr - 30 Jun. Oval Pigtoe (Pleurobema pyriforme) US: E; GA: E Occurrence; Critical Habitat; Survey period: year round, when water temperatures are above 10° C and excluding when stage is increasing or above normal. Pondberry (Lindera melissifolia) US: E; GA: E Occurrence; Survey period: flowering 1 Feb - 31 Mar or fruiting 1 Aug - 31 Oct. Green Pitcherplant (Sarracenia oreophila) US: E; GA: E Occurrence; Survey period: flowering 1 May - 30 Jun. -

Stream-Temperature Charcteristics in Georgia
STREAM-TEMPERATURE CHARACTERISTICS IN GEORGIA U.S. GEOLOGICAL SURVEY Prepared in cooperation with the GEORGIA DEPARTMENT OF NATURAL RESOURCES ENVIRONMENTAL PROTECTION DIVISION Water-Resources Investigations Report 96-4203 STREAM-TEMPERATURE CHARACTERISTICS IN GEORGIA By T.R. Dyar and S.J. Alhadeff ______________________________________________________________________________ U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 96-4203 Prepared in cooperation with GEORGIA DEPARTMENT OF NATURAL RESOURCES ENVIRONMENTAL PROTECTION DIVISION Atlanta, Georgia 1997 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director For additional information write to: Copies of this report can be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Branch of Information Services 3039 Amwiler Road, Suite 130 Denver Federal Center Peachtree Business Center Box 25286 Atlanta, GA 30360-2824 Denver, CO 80225-0286 CONTENTS Page Abstract . 1 Introduction . 1 Purpose and scope . 2 Previous investigations. 2 Station-identification system . 3 Stream-temperature data . 3 Long-term stream-temperature characteristics. 6 Natural stream-temperature characteristics . 7 Regression analysis . 7 Harmonic mean coefficient . 7 Amplitude coefficient. 10 Phase coefficient . 13 Statewide harmonic equation . 13 Examples of estimating natural stream-temperature characteristics . 15 Panther Creek . 15 West Armuchee Creek . 15 Alcovy River . 18 Altamaha River . 18 Summary of stream-temperature characteristics by river basin . 19 Savannah River basin . 19 Ogeechee River basin. 25 Altamaha River basin. 25 Satilla-St Marys River basins. 26 Suwannee-Ochlockonee River basins . 27 Chattahoochee River basin. 27 Flint River basin. 28 Coosa River basin. 29 Tennessee River basin . 31 Selected references. 31 Tabular data . 33 Graphs showing harmonic stream-temperature curves of observed data and statewide harmonic equation for selected stations, figures 14-211 . -

2020 Integrated 305(B)/303(D) List
2020 Integrated 305(b)/303(d) List - Streams Reach Name/ID Reach Location/County River Basin/ Assessment/ Cause/ Size/Unit Category/ Notes Use Data Provider Source Priority Alex Creek Mason Cowpen Branch to Altamaha Not Supporting DO 3 4a TMDL completed DO 2002. Altamaha River GAR030701060503 Wayne Fishing 1,55,10 NP Miles Altamaha River Confluence of Oconee and Altamaha Supporting 72 1 TMDL completed Fish Tissue (Mercury) 2002. Ocmulgee Rivers to ITT Rayonier GAR030701060401 Appling, Wayne, Jeff Davis Fishing 1,55 Miles Altamaha River ITT Rayonier to Altamaha Assessment 20 3 TMDL completed Fish Tissue (Mercury) 2002. More Penholoway Creek Pending data need to be collected and evaluated before it GAR030701060402 Wayne Fishing 10,55 Miles can be determined whether the designated use of Fishing is being met. Altamaha River Penholoway Creek to Altamaha Supporting 27 1 Butler River GAR030701060501 Wayne, Glynn, McIntosh Fishing 1,55 Miles Beards Creek Chapel Creek to Spring Altamaha Not Supporting Bio F 7 4a TMDL completed Bio F 2017. Branch GAR030701060308 Tattnall, Long Fishing 4 NP Miles Beards Creek Spring Branch to Altamaha Not Supporting Bio F 11 4a TMDL completed Bio F in 2012. Altamaha River GAR030701060301 Tattnall Fishing 1,55,10,4 NP, UR Miles Big Cedar Creek Griffith Branch to Little Altamaha Assessment 5 3 This site has a narrative rank of fair for Cedar Creek Pending macroinvertebrates. Waters with a narrative rank GAR030701070108 Washington Fishing 59 Miles of fair will remain in Category 3 until EPD completes the reevaluation of the metrics used to assess macroinvertebrate data. Big Cedar Creek Little Cedar Creek (at Altamaha Not Supporting FC 6 5 EPD needs to determine the "natural DO" for the Donovan Hwy) to Little area before a use assessment is made.