Trichoblastic Carcinoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Dermoscopic Features of Trichoadenoma
Dermatology Practical & Conceptual Broadening the List of Basal Cell Carcinoma Mimickers: Dermoscopic Features of Trichoadenoma Riccardo Pampena1, Stefania Borsari1, Simonetta Piana2, Caterina Longo1,3 1 Centro Oncologico ad Alta Tecnologia Diagnostica, Azienda Unità Sanitaria Locale - IRCCS di Reggio Emilia, Italy 2 Pathology Unit, Azienda Unità Sanitaria Locale - IRCCS di Reggio Emilia, Italy 3 Department of Dermatology, University of Modena and Reggio Emilia, Modena, Italy Key words: trichoadenoma, basal cell carcinoma, adnexal tumors, dermoscopy Citation: Pampena R, Borsari S, Piana S, Longo C. Broadening the list of basal cell carcinoma mimickers: dermoscopic features of trichoadenoma. Dermatol Pract Concept. 2019;9(2):160-161. DOI: https://doi.org/10.5826/dpc.0902a17 Accepted: January 10, 2019; Published: April 30, 2019 Copyright: ©2019 Pampena et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This research was supported by Italian Ministry of Health (Project Code: NET-2011-02347213). Competing interests: The authors have no conflicts of interest to disclose. Authorship: All authors have contributed significantly to this publication. Corresponding author: Riccardo Pampena, MD, Centro Oncologico ad Alta Tecnologia Diagnostica, Azienda Unità Sanitaria Locale – IRCCS, Viale Risorgimento 80, 42123, Reggio Emilia, Italy. Email: [email protected] Introduction Case Presentation A wide spectrum of skin tumors may mimic basal cell carci- Dermoscopic evaluation was performed with a contact polar- noma (BCC) on both clinical and dermoscopic appearance. ized dermatoscope (DermLite Foto, 3Gen LLC, Dana Point, Among these, adnexal skin neoplasms and in particular CA, USA) and showed a general BCC-like appearance. -

Abstract Case Synopsis
Volume 20 Number 4 April 2014 Case presentation Solitary papule over scalp 1 1 1 2 Nidhi Singh , Laxmisha Chandrashekar , Devinder Mohan Thappa , Rakhee Kar Dermatology Online Journal 20 (4): 6 1Department of Dermatology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India 2Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India Correspondence: Laxmisha Chandrashekar Associate Professor, Department of Dermatology, Jawaharlal Institute of Postgraduate Medical Education and Research Puducherry, India [email protected] Abstract Folliculosebaceous cystic hamartoma (FSCH) is a rare cutaneous hamartoma characterized by follicular, sebaceous, and mesenchymal elements. Folliculosebaceous cystic hamartoma is probably not as rare as previously thought and its inclusion in the differential diagnosis of asymptomatic skin colored papules or nodules is warranted, especially if it is present in the head and neck region. Key words: folliculosebaceous cystic hamartoma, sebaceous tumor Case synopsis A 33-year-old woman presented with an asymptomatic papule that had persisted for the past 11 years. She noticed slow growth in the size of the lesion over the past 5 years. Repeated trauma to the papule while combing her hair resulted in discomfort. Physical examination revealed a single non-tender, skin colored, firm, hairless papule of 5 x 4 x 3 mm diameter over the vertex of the scalp (Figure 1). It was excised and sent for histopathological examination (Figure 2, 3 & 4). Histopathological examination revealed a dilated follicular cystic structure with numerous sebaceous lobules radiating out from it in the dermis (Figure 2). The cyst showed a predominantly infundibular keratinization (Figure 3). This folliculosebaceous structure was surrounded by increased collagen in the dermis (Figure 2) and clefts were visible between the folliculosebaceous structures and the surrounding stroma (Figure 4). -

Cutaneous Neoplasms
torr CALIFORNIA TUMOR TISSUE REGISTRY 1 03RD SEMI-ANNUAL CANCER SEMINAR ON CUTANEOUS NEOPLASMS CASE HISTORIES 00•MODERAT.0RS: . PHILIP E. LE~0FJ', M.Q. Dir;ector O:f Oermatopafholo.gy ;Ser:Vice Associate Professor of Clinical Pathology U.C.S.F.- Elermatopa~hology San Francisco, ·californla and TIMGTH1f' H. MCG~WMON'f,, M ~D. Assistant Clinical Professor U.C~S.F. - Dermatopathology San Francisco, California December 7, 1997 Sheraton Palace Hotel San Francisco, California PLATFORM CHAIR: CLAUDE 0. BURDICK, M.D. Director of laboratory ValleyCare Health System Pleasanton, California CASE RISTORJES 10.3"" Semi-Annual Seminar (Due to in$uffient material, Case 115 is • compo~ite to two ca!ICll with an identical diagnosis, Ace. #15523 and Ace #12395.) Ca.c 1#1 - As:c 1#28070: The patient was a 12-ycaro{)ld male who had a fairly long history ofa very small bump in the scalp of the temporal area, which had recently become greally enlarged. The submitting denna!ologist mentioned that this was a soliwy lesion, with no other lesions apparent (Contributed by Prescott Rasmussen, MD.) c-111- As:c #11543: The patient was a 60-year-old Caucasian female wbo presented with a S.O em right suprapalellar subcutaneous mass which was reported to be present and gradually increasing in size for a period of approximately rn·o years. There was no history of prior trauma, and the remainder ofthe clinical history and physical findings wcze uoremarialble. An cxeisional biopsy was performed. The specimeD consisted ofa 4.S x 1.1 em elliptical segment ofeentnllly dimpled skin which surmowlted a S.3 x 4.4 x 3.6 em delicately encapsulated. -

Trichoblastoma Arising from the Nevus Sebaceus of Jadassohn
Open Access Case Report DOI: 10.7759/cureus.15325 Trichoblastoma Arising From the Nevus Sebaceus of Jadassohn Fatimazahra Chahboun 1 , Madiha Eljazouly 1 , Mounia Elomari 2 , Faycal Abbad 3 , Soumiya Chiheb 1 1. Dermatology Unit, Cheikh Khalifa International University Hospital, Mohammed VI University of Health Sciences, Casablanca, MAR 2. Plastic and Reconstructive Surgery, Cheikh Khalifa International University Hospital, Mohammed VI University of Health Sciences, Casablanca, MAR 3. Pathology, Cheikh Khalifa International University Hospital, Mohammed VI University of Health Sciences, Casablanca, MAR Corresponding author: Fatimazahra Chahboun, [email protected] Abstract Trichoblastoma is a rare benign skin adnexal tumour, belonging to the category of trichogenic tumours. The clinical and histological findings may often be confused with basal cell carcinoma, a malignant epidermal skin tumour. We report here a case of a 70-year-old man presented with a dome-shaped, dark-pigmented nodule within a yellowish hairless plaque on the scalp. The plaque had existed since childhood. However, the central pigmented nodule began appearing three months ago and enlarging gradually. The patient had no medical history. Furthermore the physical examination revealed a translucent, verrucous, and yellowish plaque, with central and pigmented nodule measuring 0.7 × 0.5 cm. Also basal cell carcinoma and trichoblastoma’s diagnosis were discussed. The patient was subsequently referred to the plastic surgery department, where he underwent a total excision. The histological examination was in favour of trichoblastoma arising from the nevus sebaceus. After 24 months of checking, no recurrence was observed. Trichoblastoma is a benign adnexal tumour. Its progression to malignant trichoblastoma (or trichoblastic carcinoma) is possible, but remains exceptional. -

The Best Diagnosis Is: H&E, Original Magnification 2
Dermatopathology Diagnosis The best diagnosis is: H&E, original magnification 2. a. adenoid cysticcopy carcinoma arising within a spiradenoma b. cylindroma and spiradenoma collision tumor c. microcysticnot change within a spiradenoma d. mucinous carcinoma arising within a spiradenoma Doe. trichoepithelioma and spiradenoma collision tumor CUTIS H&E, original magnification 100. PLEASE TURN TO PAGE 211 FOR DERMATOPATHOLOGY DIAGNOSIS DISCUSSION Amanda F. Marsch, MD; Jeffrey B. Shackelton, MD; Dirk M. Elston, MD Dr. Marsch is from the Department of Dermatology, University of Illinois at Chicago. Drs. Shackelton and Elston are from the Ackerman Academy of Dermatopathology, New York, New York. The authors report no conflict of interest. Correspondence: Amanda F. Marsch, MD, University of Illinois at Chicago, 808 S Wood St, Chicago, IL 60612 ([email protected]). 192 CUTIS® WWW.CUTIS.COM Copyright Cutis 2015. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. Dermatopathology Diagnosis Discussion Trichoepithelioma and Spiradenoma Collision Tumor he coexistence of more than one cutaneous adnexal neoplasm in a single biopsy specimen Tis unusual and is most frequently recognized in the context of a nevus sebaceous or Brooke-Spiegler syndrome, an autosomal-dominant inherited disease characterized by cutaneous adnexal neoplasms, most commonly cylindromas and trichoepitheliomas.1-3 Brooke-Spiegler syndrome is caused by germline muta- tions in the cylindromatosis gene, CYLD, located on band 16q12; it functions as a tumor suppressor gene and has regulatory roles in development, immunity, and inflammation.1 Weyers et al3 first recognized the tendency for adnexal collision tumors to present in patients with Brooke-Spiegler syndrome; they reported a patient with Brooke-Spiegler syndrome with spirad- Figure 1. -

Rippled-Pattern Sebaceoma: a Report of a Lesion on the Back with a Review of the Literature
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Fukui Repository Rippled-pattern sebaceoma: A report of a lesion on the back with a review of the literature Takahiro Kiyohara, M.D., Masanobu Kumakiri, M.D., Hiroaki Kuwahara, M.D., Atsuko Saitoh, M.D., and Shinichi Ansai, M.D. Department of Dermatology (T.K., M.K.), University of Fukui, Fukui; Division of Plastic Surgery (H.K.), Obihiro-Kousei General Hospital, Obihiro: Sapporo Institute for Dermatopathology (S.A.), Sapporo, Japan Address correspondence and reprint requests to: Takahiro Kiyohara, M.D. Department of Dermatology, University of Fukui 23-3 Shimoaizuki, Matsuoka-cho, Yoshida-gun, Fukui 910-1193, Japan Tel: +81 776 61 3111 Fax: +81 776 61 8112 e-mail: kiyo @ fmsrsa.fukui-med.ac.jp Abstract A 68-year-old Japanese man presented with a tumor that had been present for 5 to 6 years on the right back. Physical examination revealed a dome-shaped, 12x13-mm, dark red tumor. The tumor was excised with a 2 to 3-mm margin. The patient has remained free of disease during 77-months of follow-up. Microscopic examination revealed a bulb-like tumor in the dermis, contiguous with the overlying epidermis. It was composed of small, monomorphous, cigar-shaped basaloid cells in linear, parallel rows, resembling the palisading of nuclei of Verocay bodies, and presenting a rippled-pattern. There were scattered cells showing sebaceous differentiation with vacuolated cytoplasm and scalloped nuclei. There were tiny, duct-like spaces. The tumor revealed characteristics of rippled-pattern sebaceoma. -

Microcystic Adnexal Carcinoma: Review of a Potential Diagnostic Pitfall and Management
Microcystic Adnexal Carcinoma: Review of a Potential Diagnostic Pitfall and Management Lana H. McKinley, DO; Stacey Seastrom, DO; Andrew J. Hanly, MD; Richard A. Miller, DO Practice Points Microcystic adnexal carcinoma (MAC) is a rare, locally aggressive, malignant cutaneous neoplasm with pilar and eccrine gland differentiation. Microcystic adnexal carcinoma should be considered in the differential diagnosis for slow-growing tumors of the head and neck. Initial misdiagnosis of MAC is possible, as the superficial histologic findings often mimic benign follicu- lar neoplasms. Mohs micrographic surgeCUTISry is the treatment of choice for MAC. Microcystic adnexal carcinoma (MAC) is an uncom- carcinoma with syringomatous features.1 Clinically, it mon, locally aggressive cutaneous neoplasm that presents as a slow-growing, asymptomatic lesion on usually presents as a slow-growing, asymptomatic the head or neck. Microcystic adnexal carcinoma has lesion on the head or neck. Microcystic adnexal a predilection for white individuals and has a slight carcinoma frequently is misdiagnosed due to female predominance.2 It frequently is misdiagnosed its histologic appearance on superficial biopsy due to its histologic appearance on superficial biopsy specimensDo mimicking other follicularNot neoplasms. specimens Copy mimicking other follicular neoplasms. Herein, we highlight a case in which a slow- growing lesion was initially diagnosed as a tricho- Case Report adenoma following superficial biopsy; however, A 90-year-old white woman presented with a lesion on after surgical excision the pathology revealed a the left side of the upper lip of 1 year’s duration. She locally aggressive MAC. denied pain, bleeding, pruritus, or history of a similar Cutis. 2014;93:162-165. -

Sebaceous Cell Carcinoma: a Persistent Challenge in Clinical And
erimenta xp l D E e r & m l a a t c o i l n o i Journal of Clinical & Experimental l g y C f R o e l ISSN: 2155-9554 s a e n Miyamoto et al., J Clin Exp Dermatol Res 2016, 7:3 a r r u c o h J Dermatology Research DOI: 10.4172/2155-9554.1000353 Review Article Open Access Sebaceous Cell Carcinoma: A Persistent Challenge in Clinical and Histopathological Diagnosis Denise Miyamoto1*, Beatrice Wang2, Cristina Miyamoto3,4, Valeria Aoki1, Li Anne Lim4, Paula Blanco4 and Miguel N Burnier4 1Department of Dermatology, University of São Paulo Medical School, Brazil 2Department of Dermatology, McGill University, Canada 3Department of Ophthalmology, Federal University of São Paulo, Brazil 4From the Henry C. Witelson Ocular Pathology Laboratory, McGill University, Canada *Corresponding author: Denise Miyamoto, University of São Paulo Medical School, São Paulo-State of São Paulo, Brazil, Tel: 514-934-76129; E-mail: [email protected] Received date: April 25, 2016; Accepted date: May 20, 2016; Published date: May 25, 2016 Copyright: © 2016 Miyamoto D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Sebaceous cell carcinoma continues to defy clinicians and pathologists in terms of early diagnosis. The tumor may be mistaken as benign lesions such as chalazion and blepharitis, and also as malignant neoplasms, mainly basal cell carcinoma and squamous cell carcinoma. Despite advances in immunohistochemical analysis and treatment options during the last decades, morbidity and metastasis rates remain high. -

Giant Vascular Eccrine Spiradenoma Ann Dermatol Vol
Giant Vascular Eccrine Spiradenoma Ann Dermatol Vol. 23, Suppl. 2, 2011 http://dx.doi.org/10.5021/ad.2011.23.S2.S197 CASE REPORT Giant Vascular Eccrine Spiradenoma Min Ho Kim, M.D., Eujin Cho, M.D., Jeong Deuk Lee, M.D., Ph.D., Sang Hyun Cho, M.D., Ph.D. Department of Dermatology, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea Giant vascular eccrine spiradenomas (GVESs) are a rare The diagnosis of GVES was made via histopathological variant of the eccrine spiradenoma that develops from the examination. sweat gland. It is different from the eccrine spiradenoma in its larger size and greater degree of vascularity. Bleeding CASE REPORT and/or ulceration are common clinical features of this tumor, and are the reason why it is often clinically confused with a A 49-year-old woman presented with a solitary deep mass vascular or malignant tumor. Here, a rare case of GVES of 10-year duration on the right upper arm. The lesion was without bleeding or ulceration is reported. (Ann Dermatol asymptomatic, firm, and 2.5×2.5 cm in size, with a bluish 23(S2) S197∼S200, 2011) color in the overlying intact skin (Fig. 1). On physical ex- amination, there were no abnormal findings other than the -Keywords- cutaneous lesion. An excisional biopsy was performed Eccrine spiradenoma, Giant vascular eccrine spiradenoma, and the specimen was grossly hemorrhagic. The histo- Sweat gland neoplasms pathological findings of the lesion showed a large, well-circumscribed, encapsulated, multilobulated tumor in the dermis. There was extensive hemorrhage and numer- INTRODUCTION ous dilated vascular spaces containing red blood cells or pale pinkish lymph fluid in the tumor (Fig. -
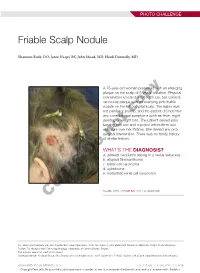
Friable Scalp Nodule
PHOTO CHALLENGE Friable Scalp Nodule Shannon Buck, DO; Jaree Naqvi, BS; John Moad, MD; Heidi Donnelly, MD A 75-year-old woman presented with an enlarging plaque on the scalp of 5 years’ duration. Physical examination revealed a 5.6×2.9-cm, tan-colored, verrucous plaque with an overlying pink friable nodule on the left occipital scalp. The lesion was not painful or pruritic,copy and the patient did not have any constitutional symptoms such as fever, night sweats, or weight loss. The patient denied prior tanning bed use and reported intermittent sun exposure over her lifetime. She denied any prior surgicalnot intervention. There was no family history of similar lesions. WHAT’S THE DIAGNOSIS? Doa. adnexal neoplasm arising in a nevus sebaceus b. atypical fibroxanthoma c. basal cell carcinoma d. cylindroma e. metastatic renal cell carcinoma CUTIS PLEASE TURN TO PAGE E20 FOR THE DIAGNOSIS Drs. Buck and Donnelly are from Dayton Skin Care Specialists, Ohio. Mr. Naqvi is from Boonshoft School of Medicine, Wright State University, Dayton. Dr. Moad is from Dermatopathology Laboratory of Central States, Dayton. The authors report no conflict of interest. Correspondence: Shannon Buck, DO, Dayton Skin Care Specialists, 3025 Governor’s Pl Blvd, Dayton, OH 45409 ([email protected]). WWW.MDEDGE.COM/DERMATOLOGY VOL. 105 NO. 1 I JANUARY 2020 E19 Copyright Cutis 2020. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Adnexal Neoplasm Arising in a Nevus Sebaceus iopsy of the lesion showed a proliferation of basa- secondary neoplasms, 88% of which were benign.2 The loid-appearing cells with focal ductal differentiation origins of these neoplasms can be epithelial, sebaceous, Band ulceration consistent with poroma (Figure 1). -

Than Skin Deep: a Case of Nevus Sebaceous Associated with Basal Cell Carcinoma Transformation
Open Access Case Report DOI: 10.7759/cureus.9386 More Than Skin Deep: A Case of Nevus Sebaceous Associated With Basal Cell Carcinoma Transformation Shauna Maty 1 , Kristen Salana 1 , Mihaela Radu 2 , Cristina Beiu 3 , Robert Hage 4 1. Dermatology, St. George's University School of Medicine, St. George, GRD 2. Dermatology, Emergency Clinical Hospital "Sf. Apostol Andrei", Constanta, ROU 3. Oncologic Dermatology, Elias Emergency University Hospital, "Carol Davila" University of Medicine and Pharmacy, Bucharest, ROU 4. Otolaryngology, St. George's University School of Medicine, St. George, GRD Corresponding author: Shauna Maty, [email protected] Abstract Nevus sebaceous is a congenital epidermal lesion that typically presents in infancy from the neck up and rarely undergoes malignant transformation. In patients who do present with malignancy, both RAS oncogene and PTCH tumor suppressor gene mutations have been implicated. We report an unusual case of nevus sebaceous in a 41-year-old male patient that developed into basal cell carcinoma on the forehead, and elaborate on the prolonged nature and unique location of its presentation. The case highlights the need for early intervention and how variable access to primary care can impact patient outcomes. We further explore the role of gene mutations in the circumstance that nevus sebaceous does become malignant, as well as pertinent differential diagnoses to consider. Categories: Dermatology, Genetics Keywords: nevus sebaceous, basal cell carcinoma, gene mutations, mosaicism, dermatology Introduction Nevus sebaceous is a type of rare congenital birthmark or skin hamartoma found in up to 0.3% of neonates that typically present from the neck up, most commonly found on the scalp. -

Trichoadenoma of the Upper Lip Gian Paolo Bombeccari, Gianpaolo Guzzi, Umberto Mariani, Andrea Gianatti, Diego Ruffoni, Franco Santoro, Francesco Spadari
CASE REPORTS SCIENTIFIC ARTICLES Stomatologija, Baltic Dental and Maxillofacial Journal, 17: 102-4, 2015 Trichoadenoma of the upper lip Gian Paolo Bombeccari, Gianpaolo Guzzi, Umberto Mariani, Andrea Gianatti, Diego Ruffoni, Franco Santoro, Francesco Spadari SUMMARY Background. Trichoadenoma of Nikolowski, who describe the first cases in 1958, is a rare and benign tumor of the hair follicle. It is well-differentiated and slowly-growing. The clinical appearance of Trichoadenoma (TA) can be similar to basal cell carcinoma or epidermal cyst. Results. We describe a 44-year-old male who was referred for nodular lesion on the upper lip and a TA was diagnosed. Oral examination showed exophytic yellow mass located between mucous membrane of the upper lip and vestibular gingiva, 1.2 per 0.8 cm. Anamnestic data was non-contributory. An excisional biopsy of the lesion was performed. Microscopically, the lesion consisted of multiple keratinous cysts lined with stratified squamous epithelium and intermingled with solid islands of basaloid cells lying within sclerotic stroma. The pathological diagnosis was TA. The surgical wound healed uneventfully. Conclusion. Because the lesion is unique, it is uncertain how aggressive or indolent the tumor might be. Therefore, the microscopical analysis is mandatory. At the best of our knowledge, this is the second case of trichoadenoma of the lip. Keywords: lip lesion, oral nodule, lip tumor, oral tumor, oral follicular hamartoma. INTRODUCTION Trichoadenoma (TA) is a rare benign tumor of than trichofolliculoma and is more differentiated the hair follicle, which was first described in 1958 by than trichoepithelioma (4). It is often apparent a dif- Nikolowsky as “organoid follicular hamartoma” (1).