Will Mcclatchey, Chairperson Thomas Hemscheidt Mark Merlin ·'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Weed Risk Assessment for Vitex Rotundifolia L. F. (Lamiaceae)
Weed Risk Assessment for Vitex United States rotundifolia L. f. (Lamiaceae) – Beach Department of Agriculture vitex Animal and Plant Health Inspection Service June 4, 2013 Version 2 Left: Infestation in South Carolina growing down to water line and with runners and fruit stripped by major winter storm (Randy Westbrooks, U.S. Geological Survey, Bugwood.org). Right: A runner with flowering shoots (Forest and Kim Starr, Starr Environmental, Bugwood.org). Agency Contact: Plant Epidemiology and Risk Analysis Laboratory Center for Plant Health Science and Technology Plant Protection and Quarantine Animal and Plant Health Inspection Service United States Department of Agriculture 1730 Varsity Drive, Suite 300 Raleigh, NC 27606 Weed Risk Assessment for Vitex rotundifolia Introduction Plant Protection and Quarantine (PPQ) regulates noxious weeds under the authority of the Plant Protection Act (7 U.S.C. § 7701-7786, 2000) and the Federal Seed Act (7 U.S.C. § 1581-1610, 1939). A noxious weed is defined as “any plant or plant product that can directly or indirectly injure or cause damage to crops (including nursery stock or plant products), livestock, poultry, or other interests of agriculture, irrigation, navigation, the natural resources of the United States, the public health, or the environment” (7 U.S.C. § 7701-7786, 2000). We use weed risk assessment (WRA)—specifically, the PPQ WRA model (Koop et al., 2012)—to evaluate the risk potential of plants, including those newly detected in the United States, those proposed for import, and those emerging as weeds elsewhere in the world. Because the PPQ WRA model is geographically and climatically neutral, it can be used to evaluate the baseline invasive/weed potential of any plant species for the entire United States or for any area within it. -

Plant Charts for Native to the West Booklet
26 Pohutukawa • Oi exposed coastal ecosystem KEY ♥ Nurse plant ■ Main component ✤ rare ✖ toxic to toddlers coastal sites For restoration, in this habitat: ••• plant liberally •• plant generally • plant sparingly Recommended planting sites Back Boggy Escarp- Sharp Steep Valley Broad Gentle Alluvial Dunes Area ment Ridge Slope Bottom Ridge Slope Flat/Tce Medium trees Beilschmiedia tarairi taraire ✤ ■ •• Corynocarpus laevigatus karaka ✖■ •••• Kunzea ericoides kanuka ♥■ •• ••• ••• ••• ••• ••• ••• Metrosideros excelsa pohutukawa ♥■ ••••• • •• •• Small trees, large shrubs Coprosma lucida shining karamu ♥ ■ •• ••• ••• •• •• Coprosma macrocarpa coastal karamu ♥ ■ •• •• •• •••• Coprosma robusta karamu ♥ ■ •••••• Cordyline australis ti kouka, cabbage tree ♥ ■ • •• •• • •• •••• Dodonaea viscosa akeake ■ •••• Entelea arborescens whau ♥ ■ ••••• Geniostoma rupestre hangehange ♥■ •• • •• •• •• •• •• Leptospermum scoparium manuka ♥■ •• •• • ••• ••• ••• ••• ••• ••• Leucopogon fasciculatus mingimingi • •• ••• ••• • •• •• • Macropiper excelsum kawakawa ♥■ •••• •••• ••• Melicope ternata wharangi ■ •••••• Melicytus ramiflorus mahoe • ••• •• • •• ••• Myoporum laetum ngaio ✖ ■ •••••• Olearia furfuracea akepiro • ••• ••• •• •• Pittosporum crassifolium karo ■ •• •••• ••• Pittosporum ellipticum •• •• Pseudopanax lessonii houpara ■ ecosystem one •••••• Rhopalostylis sapida nikau ■ • •• • •• Sophora fulvida west coast kowhai ✖■ •• •• Shrubs and flax-like plants Coprosma crassifolia stiff-stemmed coprosma ♥■ •• ••••• Coprosma repens taupata ♥ ■ •• •••• •• -

Estrogen-Like Activities in Vitex Species from China Determined by a Cell Based Proliferation Assay
ORIGINAL ARTICLES Department of Pharmacognosy1, School of Pharmacy, Second Military Medical University, Shanghai, China; School of Biomo- lecular Science2, Faculty of Sciences, Liverpool John Moores University, Liverpool, UK Estrogen-like activities in Vitex species from China determined by a cell based proliferation assay Y. Hu1, Q.-Y. Zhang1, T.-T. Hou1, H.-L. Xin1, H.-C. Zheng1, K. Rahman2, L.-P. Qin1 Received February 14, 2007, accepted March 29, 2007 Prof. Lu-Ping Qin, Departement of Pharmacognosy, School of Pharmacy, Second Military Medical Uni- versity, 325 Guohe Road, Shanghai 200433, China [email protected], [email protected] Pharmazie 62: 872–875 (2007) doi: 10.1691/ph.2007.11.7542 Ethanolic extracts of four Chinese medicinally used Vitex species were selected and tested for their estrogen-like activities, using an ERa-positive MCF-7 cell based proliferation assay (E-screen assay) and cell cycle analysis (flow cytometry). Vitex negundo displayed the highest estrogenic-like activity, and could be useful in hormone replacement therapy (HRT). 1. Introduction 2. Investigations, result and discussion Phytoestrogens are plant-derived compounds with estro- In the E-Screen assay using MCF-7 cells, the proliferative genic or antiestrogenic properties (Umland et al. 2000). effect of the extracts relative to that of estradiol (1 nM, They seem to be interesting by their possible use as al- 100%) is expressed as Relative Proliferative Effect (RPE) ternative medicines for treating hormonal disorders such (Fig. 1). The results clearly indicate that the extracts from as in hormone replacement therapy (HRT). Thus, phy- V. rotundifolia and V. negundo were able to significantly toestrogens could be beneficial in treatment of the symp- stimulate MCF-7 cell proliferation at concentrations of toms of menopause and hence help to prevent bone re- 50 mg/mL to100 mg/mL (P < 0.01). -

Identification De Polyphénols, Évaluation De Leur Activité Antioxydante Et Étude De Leurs Propriétés Biologiques François Muanda Nsemi
Identification de polyphénols, évaluation de leur activité antioxydante et étude de leurs propriétés biologiques François Muanda Nsemi To cite this version: François Muanda Nsemi. Identification de polyphénols, évaluation de leur activité antioxydante et étude de leurs propriétés biologiques. Biologie végétale. Université Paul Verlaine - Metz, 2010. Français. NNT : 2010METZ011S. tel-01752680 HAL Id: tel-01752680 https://hal.univ-lorraine.fr/tel-01752680 Submitted on 29 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D'autre part, toute contrefaçon, plagiat, reproduction illicite encourt une poursuite pénale. Contact : [email protected] LIENS Code de la Propriété Intellectuelle. articles L 122. 4 Code de -

Vitex Rotundifolia L.F
NEW YORK NON -NATIVE PLANT INVASIVENESS RANKING FORM Scientific name: Vitex rotundifolia L.f. USDA Plants Code: VIRO80 Common names: Roundleaf chastetree, beach vitex, chasteberry, monk's pepper Native distribution: Asia (China, Japan), India, Sri Lanka, Mauritius, Australia, Pacific Islands (inlcuding Hawaii) Date assessed: 3 June 2009; edited August 19, 2009 Assessors: Steve Glenn, Gerry Moore Reviewers: LIISMA SRC Date Approved: August 19, 2009 Form version date: 3 March 2009 New York Invasiveness Rank: High (Relative Maximum Score 70.00-80.00) Distribution and Invasiveness Rank ( Obtain from PRISM invasiveness ranking form ) PRISM Status of this species in each PRISM: Current Distribution Invasiveness Rank 1 Adirondack Park Invasive Program Not Assessed Not Assessed 2 Capital/Mohawk Not Assessed Not Assessed 3 Catskill Regional Invasive Species Partnership Not Assessed Not Assessed 4 Finger Lakes Not Assessed Not Assessed 5 Long Island Invasive Species Management Area Not Present Moderate 6 Lower Hudson Not Assessed Not Assessed 7 Saint Lawrence/Eastern Lake Ontario Not Assessed Not Assessed 8 Western New York Not Assessed Not Assessed Invasiveness Ranking Summary Total (Total Answered*) Total (see details under appropriate sub-section) Possible 1 Ecological impact 40 ( 40 ) 37 2 Biological characteristic and dispersal ability 25 ( 25 ) 20 3 Ecological amplitude and distribution 25 ( 25 ) 4 4 Difficulty of control 10 ( 10 ) 8 Outcome score 100 ( 100 )b 73.00 a † Relative maximum score 73.00 § New York Invasiveness Rank High (Relative Maximum Score 70.00-80.00) * For questions answered “unknown” do not include point value in “Total Answered Points Possible.” If “Total Answered Points Possible” is less than 70.00 points, then the overall invasive rank should be listed as “Unknown.” †Calculated as 100(a/b) to two decimal places. -
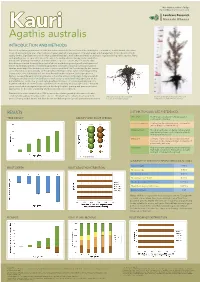
Agathis Australis
Mike Marden and Chris Phillips [email protected] KKauriauri Agathis australis INTRODUCTION AND METHODS Reasons for planting native trees include the enhancement of plant and animal biodiversity for conservation, establishment of a native cover on erosion-prone sites, improvement of water quality by revegetation of riparian areas and management for production of high quality timber. Signifi cant areas of the New Zealand landscape, both urban and rural, are being re-vegetated using native species. Many such plantings are on open sites where the aim is to quickly achieve canopy closure and often includes the planting of a mixture of shrubs and tree species concurrently. Previously, data have been presented showing the potential above- and below-ground growth performance of eleven native plant species considered typical early colonisers of bare ground, particularly in riparian areas (http://icm.landcareresearch.co.nz/research/land/Trial1results.asp). In this current series of posters we present data on the growth performance of six native conifer (kauri, rimu, totara, matai, miro, kahikatea) and two broadleaved hardwood (puriri, titoki) species most likely to succeed the early colonising species to become a major component in mature stands of indigenous forest (http://icm.landcareresearch.co.nz/research/land/Trial2.asp). Data on the potential above- and below-ground early growth performance of colonising shrubby species together with that of conifer and broadleaved species will help land managers and community groups involved in re-vegetation projects in deciding the plant spacing and species mix most appropriate for the scale of planting and best suited to site conditions. Data are from a trial established in 2006 to assess the relative growth performance of native conifer and broadleaved hardwood tree species. -

31494 I.Indd
Estudo citológico em Aegiphila sellowiana, Vitex montevidensis e Citharexylum... 101 Estudo citológico em Aegiphila sellowiana, Vitex montevidensis e Citharexylum myrianthum da bacia do Rio Tibagi, Paraná, Brasil Priscila Mary Yuyama, Alba Lúcia Cavalheiro & André Luís Laforga Vanzela Universidade Estadual de Londrina, Departamento de Biologia Geral, Centro de Ciências Biológicas – CCB, Laboratório de Biodiversidade e Restauração de Ecossistemas, Caixa Postal 6001, 86051-990, Londrina, PR, Brasil. [email protected] Recebido em 10.VII.2008. Aceito em 26.V.2010. RESUMO - Lamiaceae e Verbenaceae correspondem às principais famílias da ordem Lamiales distribuídas nas regiões tropicais e temperadas do mundo. Neste trabalho foram feitas análises citológicas de Aegiphila sellowiana Chamisso, Vitex montevidensis Chamisso e Citharexylum myrianthum Chamisso, coletadas em diferentes regiões da bacia do Rio Tibagi, Paraná, Brasil. Os resultados mostraram que A. sellowiana apresenta 2n = 42 cromossomos, maiores do que os de V. montevidensis (2n = 34) e os de C. myrianthum (2n = ca.104), esta com os menores. Porém, as três espécies apresentam núcleos interfásicos do tipo arreticulado a semi-reticulado e padrão de condensação profásico proximal, sem evidências de variação intraespecífi ca ou diferenças interespecífi cas. No entanto, nossos resultados indicam que, do ponto de vista citológico (número e tamanho dos cromossomos), indivíduos das três espécies podem ser identifi cados corretamente e assim, suas sementes serem utilizadas, com segurança, em programas de restauração ambiental na bacia do rio Tibagi. Palavras-chave: cromossomos, número, tamanho, núcleos interfásicos. ABSTRACT - Cytological study of Aegiphila sellowiana, Vitex montevidensis and Citharexylum myrianthum from Tibagi River Basin, Paraná, Brazil. Lamiaceae and Verbenaceae are the main families in order Lamiales distributed in all tropical and temperate regions of the world. -

Download a Nomination Form from the NZPCN Website Here
TRILEPIDEA Newsletter of the New Zealand Plant Conservation Network NO. 156 Message from the President November 2016 Kia ora koutou, Deadline for next issue: Aft er the last AGM we have had a change in the make-up of the NZPCN council. I’d Thursday 15 December 2016 like to introduce myself as newly elected president and Matt Ward as newly elected SUBMIT AN ARTICLE Secretary. Both Matt and I have been a part of the NZPCN council for several years TO THE NEWSLETTER and are looking forward to continuing in our new roles. Sarah Beadel has stepped Contributions are welcome aside aft er three years as president, but remains on the council. I’d like to thank Sarah to the newsletter at any time. The closing date for for her excellent leadership over the last three years; I’m looking forward to working articles for each issue is with the rest of the council over the next year. Our focus in the short term is on approximately the 15th of fi nalising the Network’s strategy for the next fi ve years, investigating a new ‘back end’ each month. for our website and organising the next NZPCN conference in late 2017. Articles may be edited and used in the newsletter and/ Our current council consists of: or on the website news page. President: Rewi Elliot The Network will publish almost any article about Secretary: Matt Ward plants and plant conservation Treasurer: Nicky Oliver-Smith with a particular focus on the General member: John Barkla plant life of New Zealand and Oceania. -

RESEARCH ARTICLE Vitex Rotundifolia Fractions Induced
DOI:10.31557/APJCP.2019.20.12.3555 Vitex Rotundifolia Fractions Induced Apoptosis in Human Breast Cancer T-47D Cell Line RESEARCH ARTICLE Editorial Process: Submission:09/05/2018 Acceptance:11/29/2019 Vitex Rotundifolia Fractions Induced Apoptosis in Human Breast Cancer T-47D Cell Line via Activation of Extrinsic and Intrinsic Pathway Gul-e-Saba Chaudhry1*, Rehmat Jan1, Muhammad Naveed Zafar2, Habsah Mohammad1, Tengku Sifzizul Tengku Muhammad1* Abstract Objective: Breast cancer is the most frequently diagnosed cancer worldwide. The main objective of the present study was to evaluate the cytotoxic effects and mechanism of cell death induced by the extract and fractions of Vitex rotundifolia (leaves) in breast cancer cell line, T-47D. Methods: The cytotoxicity activity was measured using MTS assay. The mode of cell death was analysed by early (phosphatidylserine externalization) and late apoptosis (DNA fragmentation). The caspases 8, 9, 3/7 and apoptotic proteins bax, bcl-2 study were done by western blot and ELISA method. Results: The methanol extract was found to inhibit 50% growth of T-47D cells at the concentration of 79.43µg/ ml respectively after 72hr. From seven fractions, fraction F1, F2 and F3 produced cytotoxicity effects in T-47D cell line with IC50 (72hr) < 30µg/ml. The results obtained by Annexin V/PI apoptosis detection assay and TUNEL assay suggest that active fractions of Vitex rotundifolia induced early and late apoptosis (DNA fragmentation) in T-47D cell line. Moreover, western blot analysis and Caspase GloTM luminescent assay demonstrated that fractions F2 and F3 triggered apoptotic cell death via activation of caspases -8, -9 and -3/7 and up-regulation of Bax and down-regulation of Bcl-2 protein. -

Vitex Lucens)
03 Backyard Planting Programme Conservation Volunteers NZ Pūriri (Vitex lucens) Pūriri is a large, long-lived tree with some of the Size biggest flowers of any New Zealand tree and aided 20m tall in pollination by our birds. Large, pink flowers followed by marble sized, bright Distribution & Habitat red fruits drape the tree for most of the year. The Found from the North Cape to Waikato. You will fruiting period normally commences early winter usually see them in paddocks where they have and extends into mid-spring. been left to shade cattle. Occurs mostly in coastal and lowland forests. Fast growing tree Pūriri have the incredible ability to resprout and that will do well in most habitats. continue growing from where they have fallen over or supposedly senesced (died). Species it attracts You will see this beautiful gnarled tree studded Fruits are loved by Kererū, who are the only with the Pepetuna (Pūriri moth) burrows that are birds able to swallow the berries whole and predated on by Ruru (Morepork). With both species distribute them. Flowers also provide nectar being nocturnal you may need a torch to see for Tūī, Korimako (Bellbird), Tauhou (Silvereye). this hive of activity. Medicinal Properties Pūriri, being a hardwood with little buoyancy, was used in the construction of hīnaki (eel pots) along Leaves are boiled down to treat back ache. with many a fence post still in the ground today. Where to plant in your garden: Pūriri is a large tree with a wide root system so is best suited Pepe-tuna nunui Large pepe-tuna (pūriri moth) to large gardens. -

NOTES on AGATHIS AUSTRALIS. by C
NOTES ON AGATHIS AUSTRALIS. By C. T. SANDO. Introduction.—Much has been published about our kauri (Agathis australis) and it is now generally recognised as one of the finest coniferous trees of the world. The New Zealand species is one of a genus of ten—the most important being— A. Palmerstoni ... (Queensland) A. microstachya ... (Queensland) A. robusta ... (Queensland and Philippine Islands) A. vitiensis * ... (Fiji) A. alba ... (Malaya, Sumatra, Java, Celebes, Borneo, and Philippine Islands) A. lanceolata ... (New Caledonia) A. macrophylla ... (Solomon Islands) The value of Agathis australis was first recognised before 1800 by which time a considerable trade in kauri spars had sprung up between Australia and New Zealand. Later, naval-store ships were sent out from England for cargoes and from that time on kauri has increased in value. Up till a short time ago it was used for building and interior furnishing, but now the limited supplies demand a price far too high for these purposes so that its present uses have been confined to carriage building, cabinet-making, and general joinery, vats, tubs, and other special purposes. Wanton waste has so depleted out kauri areas that now, more than ever it is forced upon us, the necessity of making very serious attempts at conservation of the remaining supplies and propagation of this tree for scenic purposes, and, if economic conditions warrant it, for timber production. Geographical, Climatic, and Edaphic Range.—Kauri occurs in the northern parts of the North Island except in the extreme northern peninsula. It is found from Ahipara and Mangonui in the north as far south as the Bay of Plenty on the East Coast and Kawhia Harbour on the west, i.e. -

A Basis for the Management of New Zealand Kauri (Agathis Australis (D
A BASIS FOR THE MANAGEMENT OF NEW ZEALAND KAURI (AGATHIS AUSTRALIS (D. DON) LINDL.) FOREST J. C. HALKETT* ABSTRACT The area of primeval kauri (Agathis australis (D.Don) Lindl.) forest has been substantially reduced. There now exists a significant second-crop resource. Kauri forests are important ecosystems which are valuable for timber production plus non-wood values and benefits. The management potential of kauri has been indicated by research. This and other considerations resulted in a revised kauri management policy being introduced in 1973. Sustained yield management is prescribed for timber production zones. This necessitates a knowledge of the ecological character istics and an appreciation of growth dynamics, stand structures and extent of the resource. The productivity of second-crop stands suggests that there exists the opportunity for increasing the present cut. , ^fil To preserve its biological features areas of kauri forests have been set aside in scientific reserves. Re-afforestation is primarily aimed at rehabilitating disturbed forest and silvicultural tending is designed to promote the growth of regenerating areas. Harvesting in mature stands has been suspended and for environmental, engineering and management reasons helicopter logging is practised in second-crop stands. The principal function of kauri management will continue to be to protect intrinsic forest values. However, it will also prescribe activities aimed at producing a small perpetual yield of timber. INTRODUCTION New Zealand kauri {Agathis australis (D.Don) Lindl.) is the southernmost species of the genus. The other 12 species of Agathis are concentrated in the tropics (Whitmore, 1979; Bowen and Whitmore, 1980). Before the colonisation of New Zealand by Europeans, in the early 19 th century, there was in the vicinity of 1.5 million hec- *New Zealand Forest Service, P.O.