Interactions Between Beetle Larvae and Their Termite Hosts (Coleoptera; Isoptera, Nasutitermitinae) by Cassiano S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
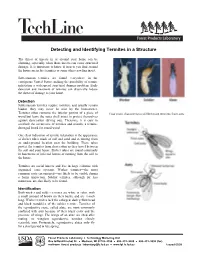
Termites in a Structure
Detecting and Identifying Termites in a Structure The threat of insects in or around your home can be alarming, especially when those insects can cause structural damage. It is important to know if insects you find around the house are in fact termites or some other crawling insect. Subterranean termites are found everywhere in the contiguous United States, making the possibility of termite infestation a widespread structural damage problem. Early detection and treatment of termites can drastically reduce the threat of damage to your home. Detection Subterranean termites require moisture and usually remain hidden—they may never be seen by the homeowner. Termites often consume the interior portion of a piece of Four main characteristics differentiate termites from ants: wood but leave the outer shell intact to protect themselves against desiccation (drying out). Therefore, it is easy to overlook the occurrence of termites and mistake a termite- damaged board for sound wood. One clear indication of termite infestation is the appearance of shelter tubes made of soil and sand and stemming from an underground location near the building. These tubes protect the termites from desiccation as they travel between the soil and your house. Shelter tubes are found commonly in basements of infected homes or running from the soil to the house. Termites are social insects and live in large colonies with organized caste systems. Worker termites—the most common caste encountered—are likely to be visible during a home inspection. Soldier termites, although far less numerous, are also likely to be found. Identification Both worker and soldier termites are white in color, with a small amount of brown on their backs, and are ¼-inch long. -

03 Literature Review
- Literature Review: - Sr. No .1 - Name of the Author : P.G. Koehler and C. L. Tucker - Name of the Article : Subterranean Termite - Pages: 7 Nos. - Date of Published : April 1993 Revised in 2003 University of Florida, IFAS, Extention No. ENY 210 - Journal Name : Entomology and Nematology, Florida Cooperative Service - Review: Subterranean Termite, Dampwood and Drywood Termite are the main three types of termites . Subterranean Termites are the most destructive and it attacks structures by building tubes from nest to wood structures. They feed on wood which contain cellulose which is digested by them by protozoa and convert cellulose into usable food. The nest in the soil contains moisture to protect them from low humidity and predation and they build mud tunnels or tubes to reach wood. At swarming stage the broken wings are located at light are hence they are attracted to light. No wood contact with soil, ventilation, regular inspection can avoid the entry of termite and for effective control for Subterranean termite, Pre and post treatment methods for new and for existing structures at different stages with repellent and non repellent insecticides as required with the additional control method of using Termite Bait. - Sr. No . 2 - Name of the Author : Matthew R. Tarver, Christopher B. Florane, Dunhua Zhang, Casey Grimm and Alan R. Lax - Name of the Article : Methoprene and temperature effects on caste differentiation and protein composition in the Formosan Subterranean termite, Coptotermesfomosanus - Pages: 10 Nos. - Date of Published : December 2011 - Journal Name : Journal of Insect Science, Vol. 12/ article 18 - Review: Worker to soldier differentiation is modulated by temperature i.e. -

Research in Biological Control of the Formosan Subterranean Termite Cai Wang Louisiana State University and Agricultural and Mechanical College, [email protected]
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2014 Research in Biological Control of the Formosan Subterranean Termite Cai Wang Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Entomology Commons Recommended Citation Wang, Cai, "Research in Biological Control of the Formosan Subterranean Termite" (2014). LSU Doctoral Dissertations. 598. https://digitalcommons.lsu.edu/gradschool_dissertations/598 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. RESEARCH IN BIOLOGICAL CONTROL OF THE FORMOSAN SUBTERRANEAN TERMITE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Cai Wang M.S., Chinese Academy of Science, 2010 B.S., Huazhong University of Science and Technology, 2007 August 2014 ACKNOWLEDGMENTS I would like to express my sincerest appreciation to my major professor, Dr. Gregg Henderson, a very important person in my life. I am very impressive for his meticulous attitude for scientific research. I benefited greatly from his valuable and illuminating suggestions for my research. It is also very touching for Dr. Henderson’s patience for my preliminary and sometimes “crazy” ideas. Also, he always could “see” what I ignored. For example, when I unintentionally talked about an observation that soldier and worker termites run in different directions after disturbance, he immediately pointed out the potential value to continue studying this and gave me valuable suggestions in the experiment. -

Totally Termites (Grades 3 – 5)
Totally Termites (Grades 3 – 5) Lesson Overview Students will explore the world of termites. This lesson includes a close-up look at termite specimens, special termite adaptations and insect anatomy. Students will also learn about property risks associated with termites, and how pest control professionals manage termite problems. Correlation with National Science Teachers Association (NSTA) § History and Nature of Science: Content Standard G: Science as a human endeavor § Life Science: Content Standard C: The characteristics of organisms § Science as Inquiry: Content Standard A: Abilities necessary to do scientific inquiry § Science in Personal and Social Perspectives: Content Standard F: Types of Resources, Changes in Environments, Science and Technology in Local Challenges Key Concepts: Vocabulary Words: § Termites in the home are animals that are out of Adaptation place; they are considered pests. § Termites are amazing insects; they have unique Insect mouthparts, a special adaptation, that helps them Mouthpart survive. § Termites have specific body features that make them Pest insects. Pest Management § Termite behavior is influenced by hunger. Risk Specimen Skills Learned: Subterranean Termite Discussion Cause/Effect Communication Social Classification Appreciation Recognition Observation www.pestworldforkids.org page 1 of 10 Totally Termites (Grades 3 – 5) Getting Ready: Estimated Time: § Preparation: 20 Minutes § Lesson: 45 – 60 Minutes Materials: § Find specimens of the Subterranean Termite: Winged Reproductive Swarmers and Workers (see Additional Resources section for termite sources) § Hand Lenses (one per student is ideal) § Termite Fact Sheet § Termite Anatomy Sheet Preparation: § Arrange for and gather termite specimens (see Resources). One specimen per student is ideal. § Gather hand lenses (one per student is ideal). § Invite a termite control specialist into the classroom as a guest speaker. -

Social Insects: Bees, Wasps, Ants and Termites
Enrico Bonatti E.d.T. Outreach Practicum 11/11/2011 Social Insects: Bees, Wasps, Ants and Termites Teacher Resource Guide Before one understands what ‘social’ insects are it is first important to understand what an insect is in the first place. Insects are small arthropods with six legs and usually one or two pairs of wings; they are placed in the class Insecta or Hexapoda. The have an exoskeleton divided in three parts; head, thorax and abdomen. What are Social Insects? The eusocial, or most social of the insects, are the bees, wasps, ants, and termites. The bees, wasps, and ants are in the Order Hymenoptera, while the termites are in the Order Isoptera. All of the ants and termites are eusocial, while both the bees and wasps have many species that live solitary lives. Certain traits characterize the eusocial insects: -Reproductive division of labor: this means that the queen reproduces almost exclusively while other members of the colony specialize on different tasks. -Cooperative brood care: this means that contrastingly to many different animals, social insects all tend the brood together indiscriminately of whose offspring it is. -In the ants and termites there are castes that carry out different functions necessary for the survival of the colony depending on the size or age of the insect they carry different functions Social insects are organized into different ‘castes’, these are characterized by specialized roles (queen, soldiers, workers and such). Usually there is a queen that produces a lot of offspring and apart from her, many other sterile daughters (workers) that depending on their age or structure carry out specific tasks in or around the colony. -

Drywood Termite Biology, Identification, and Control
ALABAMA A&M AND AUBURN UNIVERSITIES Drywood Termite Biology, Identification, and Control ANR-1170 rywood termites are tabolism (of wood) or through Identification moisture in their environments. primitive termites Identifying drywood termites Some drywood termites make whose damage often is a rather difficult process. As colonies in hot, dry areas such goes unnoticed by with all termite species, the ma- Dhomeowners. They are similar to as southern California, where the jority of the nest is composed of high temperatures and low hu- the familiar subterranean ter- worker individuals. It is a com- 1 midity reduce the available mites . Drywood termites belong mon misconception that size of water from the environment. to the family Kalotermitidae. the worker will indicate whether Since these termites live within Their ecology and behavior are the termite is a drywood or sub- their food, they must find ways distinctly different from the sub- terranean. While many drywood to remove waste from their terranean termites, a fact which species have larger workers than colonies. Drywood termites alters their monitoring and con- do subterranean termites, such make small holes in the wood trol procedures from those meth- as Incisitermes spp., other dry- they infest and occasionally kick ods used for standard subter- wood species have much smaller out fecal pellets or frass. Piles of ranean termites. workers (ex. Cryptotermes spp.). the pellets usually accumulate There is no identification key under these openings. These available for worker termites. Biology dry, smooth, and often powdery- Soldier termites and alates are Drywood termites form looking pellets are very charac- the only types of termites that colonies in a similar manner to teristic of the presence of dry- can be accurately identified. -

Bulletin 24 Is It Active Or Not.Pub
Clemson University Department of Pesticide Regulation Is It Active or Not, And How Do You Know? A Guide for Applicators Each year, the Department of Pesticide Regulation investigates concerns and questions about wood-destroying organism (WDO) activity. Determining activity is both an art and a science. It requires good judgment and a knowledge of the insect’s life history. Sec- tion 27-1085 of the Rules and Regulations for the Enforcement of the SC Pesticide Con- trol Act details criteria for determining activity of several wood-destroying organisms. This bulletin will cover determining the activity of wood infesting beetles, drywood ter- mites, and subterranean termites. Department of The term “powder post beetle” is used collectively to describe three different families of beetles: anobiids, lyctids (true powder post beetles), and bostrichids (false powder post Pesticide Regulation beetles). While there are differences in their biology and the type of wood they attack, 511 Westinghouse Road their damage appears similar. Pendleton, SC 29670 Most of the major beetle pests of wood start their destruction by laying eggs in holes, pores, or in cracks in the wood. After hatching, the tiny grubs bore into the wood, where 864.646.2150 they remain until they complete their development and emerge as adults, then the larvae www.clemson.edu/dpr eat the wood for energy. The larvae will leave a material called frass in their tunnels. Frass is loosely defined as the sawdust-like powder that comes out of the south end of a north-bound larvae. As you might expect, the consistency and appearance of frass var- ies from species to species of beetle. -

Termites—An Identification Guide
Cooperative Extension Service Household and Structural Pests Feb. 1999 HSP-1 Hawaii’s Termites—An Identification Guide awaii’s year-round warm weather has allowed Termite basics Hmany introduced insect pests to take hold, and the Hawaii’s termite species are all able to use wood as their seven species of termite found here are among these im food source with the help of microorganisms in their migrants. The most destructive termites are the Formosan digestive systems. The Formosan subterranean termite subterranean termite and the West Indian drywood ter lives primarily underground and moves up into struc mite, both of which arrived during the past 100 years tures or trees to feed. Drywood and dampwood termites and are now common throughout the state. They cause live in wood at or above ground level. more than $100 million damage per year to structures. Dampwood termites live in wet and rotting wood The other five termite species are not as great an and get all of the water they need from their food. economic threat. They are pests of trees and only occa Drywood termites use water produced from the diges sionally infest structures. Three of them, the western tion of dry wood and do not require an external source drywood, Indo-Malaysian drywood, and Pacific damp of moisture. Subterranean termites live underground and wood termites, have arrived since the 1980s, and so far obtain water from the soil around them, but they must their distribution is not extensive. But because these re venture out of this ideal environment to find food. -

Survey of the Termites (Isoptera) Of
SURVEY OF THE TERMITES (ISOPTERA) OF OKLAHOMA AND INFLUENCE OF ARTIFICIAL GUIDELINES IN SOIL ON CHANNELING FORAGING SUBTERRANEAN TERMITES By KENNETH S. BROWN Bachelor of Science Missouri Southern State College Joplin, Missouri 2000 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE December, 2002 SURVEY OF THE TERMITES (ISOPTERA) OF OKLAHOMA AND INFLUENCE OF ARTIFICIAL GUIDELINES IN SOIL ON CHANNELING FORAGING SUBTERRANEAN TERMITES Thesis Approved: ~~~ Dean of the Graduate College 11 PREFACE Chapter I of this thesis introduces research I conducted including the main objectives. Chapter II is a literature review focusing on behavior and control of subterranean termites. with special attention to the genus Reticulitermes (Isoptera: Rhinotermitidae). Chapters III and IV are formal manuscripts ofthe research I conducted during my M. S. program and are written in compliance with the publication policies and guidelines of the Entomological Society of America. None of the work put in to this thesis or toward the completion of this degree would have been possible without my Lord and Savior Jesus Christ through whom all things are possible. My work would also not have been possible without the friendship, love, and support of my wife Katie whose unselfish sacrifices while working to support me during the pursuance of this degree can only be expressed as a blessing. I would like to express my sincere appreciation to Dr. Brad Kard and Mr. Mike Doss for their friendship, guidance, and assistance throughout my project. I would also like to thank Drs. -

Genetic Mechanisms Underlying the Evolutionary Success of Eusocial Insects
insects Review (Epi)Genetic Mechanisms Underlying the Evolutionary Success of Eusocial Insects Kayli R. Sieber 1 , Taylor Dorman 1, Nicholas Newell 1 and Hua Yan 1,2,* 1 Department of Biology, University of Florida, Gainesville, FL 32611, USA; kayli.sieber@ufl.edu (K.R.S.); taylor.dorman@ufl.edu (T.D.); nicholas.newell@ufl.edu (N.N.) 2 Center for Smell and Taste, University of Florida, Gainesville, FL 32611, USA * Correspondence: hua.yan@ufl.edu; Tel.: +1-352-273-4983 Simple Summary: Social insects, namely ants, bees, and termites, are among the most numerous and successful animals on Earth. This is due to a variety of features: highly cooperative behavior performed by colony members and their specialization on a variety of tasks. Diverse physiological and behavioral specializations are regulated not only by the genetic system, but also by the epige- netic system which alters gene expressions without modifying the genetic code. This review will summarize recent advancements in such studies in eusocial insects. Abstract: Eusocial insects, such as bees, ants, and wasps of the Hymenoptera and termites of the Blattodea, are able to generate remarkable diversity in morphology and behavior despite being genetically uniform within a colony. Most eusocial insect species display caste structures in which reproductive ability is possessed by a single or a few queens while all other colony members act Citation: Sieber, K.R.; Dorman, T.; as workers. However, in some species, caste structure is somewhat plastic, and individuals may Newell, N.; Yan, H. (Epi)Genetic switch from one caste or behavioral phenotype to another in response to certain environmental cues. -

AMDRO 100530435 Quick Kill Carpenter Bee, Ant & Termite Foam
20 oz. DSC PS 8.75”W x 6.875”H .125” COPY FREE AREA 87093-7-90098_AMDRO Quick Kill Indoor Outdoor Carpenter Bee, Ant & Termite Killer Foam_20180108_25_90098_.pdf E IINSECTS KILLS: Termites* (including subterranean, drywood, dampwood and arboreal), PEEL KIILLS THE HERE AT YOU SEE & Wood Destroying Insects (Powder Post Beetles, Old House Borer, Wharf Borer), THAT ON’T Ants (Including: Argentine, Ghost, White-Footed, foraging Carpenter; Excluding: ONES YOU DON’T THE O Fire, Harvester, Leaf cutter and Pharaoh), Carpenter Bees. FOR USE IN AND AROUND STRUCTURES AND LIMITED OUTDOOR SPOT TREATMENTS: Apartments, Homes, Hobby Greenhouses, Interiorscapes. * Not a substitute for mechanical alteration, soil or foundation treatment. Kills Carpenter Bees, Carpenter Ants, Termites* (including subterranean, drywood, dampwood and arboreal) and other listed wood-damaging insects Where to Use For use in and around structures and limited outdoor spot treatments where wood- damaging insects are seen or suspected How to Use Insert applicator straw into nozzle and spray to apply where insects to be treated are seen or suspected INDOOR/OUTDOOR FIRST AID If swallowed: • Call a poison control center or doctor immediately for treatment advice. Carpenter Bee, • Have a person sip a glass of water if able to swallow. .125” COPY FREE AREA • Do not induce vomiting unless told to do so by a poison control center or doctor. Ant & Termite • Do not give anything by mouth to an unconscious person. KILLER FOAM If inhaled: • Move person to fresh air. • If person is not breathing, call 911 or an ambulance, then give artificial respiration, preferably by mouth-to-mouth, if possible. -

Termite Swarms How Does Your Garden Grow?
PestGazette SPRING 2012 How Does Your Garden Grow? Consider this Before Digging in Your Flower Beds ... ave you ever stopped to consider that what protection in the dryer months of the year. Research you do in your plant beds, especially up evidence supports the fact that termites are attracted H close to your home’s foundation, may im- to both increased thermal and moisture gradients. In pact the termite activity in and around your home? lay terms, this means that termites will follow and As you dig and plant (or even have construc- cue in on increased warmth and water areas near a tion done) near the foundation of your home, you home. If you are going to use mulch in your garden may be disrupting a termite-treated zone or barrier near your home, rake it away from the home’s foun- that you have paid for to prevent a termite infesta- dation and the treated zone; you may also want to tion. Always keep this in mind before you dig! consider using hardwood over softwood mulch, as Many homeowners love to mulch their yards. softwoods have been shown to be more attractive And there are a variety of mulch brands out there to termites. Hardwood mulch is harder to find, but including pine straws and wood/bark mulch. Mulch may be worth the effort in the long run. is meant to insulate and keep plants warm in the If for any reason you should suspect termite winter, and then provide moisture retention and activity, don’t forget to give us a call! Have to Say Termite Swarms ne of the most visible signs of a termite infestation is the presence of fly- ing termites, sometimes called swarmers.