Lecture 7: Extrinsic Semiconductors - Fermi Level
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Iii-Nitride Ultraviolet Light-Emitting Diodes: Approaches for the Enhanced Efficiency
Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept a copying order if, in its judgment, fulfillment of the order would involve violation of copyright law. Please Note: The author retains the copyright while the New Jersey Institute of Technology reserves the right to distribute this thesis or dissertation Printing note: If you do not wish to print this page, then select “Pages from: first page # to: last page #” on the print dialog screen The Van Houten library has removed some of the personal information and all signatures from the approval page and biographical sketches of theses and dissertations in order to protect the identity of NJIT graduates and faculty. ABSTRACT III-NITRIDE ULTRAVIOLET LIGHT-EMITTING DIODES: APPROACHES FOR THE ENHANCED EFFICIENCY by Moulik Patel III-nitride ultraviolet (UV) light-emitting diodes (LEDs) offer marvelous potential for a wide range of applications, including air/water purification, surface disinfection, biochemical sensing, cancer cell elimination, and many more. III-nitride semiconductor alloys, especially AlGaN and AlInN, have drawn significant attention due to their significant advantages that include environmental-friendly material composition, compact in size, longer lifetime, low power consumption, and tunable optical emission. -

Sudan University of Science and Technology College of Graduate Studies
Sudan University of Science and Technology College of Graduate Studies Increasing The Electrical Conductivity of the semiconductor by Doping and Heating زﯾﺎدة اﻟﻤﻮﺻﻠﯿﺔ اﻟﻜﮭﺮﺑﯿﺔ ﻻﺷﺒﺎه اﻟﻤﻮﺻﻼت ﺑﺎﻟﺘﺸﻮﯾﺐ و اﻟﺤﺮارة Thesis Submitted For Partial Fulfillment of the Requirement for Degree of Master in Physics Prepared by : Seddega Mohammed Noor Diab Supervised by : Dr. Ahmed ELhassan ELfaki December 2016 1 اﻵﯾــــــــــــــــــﺔ ﻗﺎل ﺗﻌﺎﻟﻰ: ﺑﺳم اﻟﻠﮫ اﻟرﺣﻣن اﻟرﺣﯾم ﯾُﻜَﻠّ ِﻒُ اﻟﻠﱠﮫُ (ﻧَﻔْﺴًﺎ ﻻ َإِﻻ ﱠ وُﺳْﻌَﮭَﺎ ﻟَﮭَﺎ ﻣَﺎ ﻛَﺴَﺒَﺖْ و َﻋَﻠَﯿْﮭَﺎ ﻣَﺎ اﻛْﺘَﺴَﺒَﺖْ رَﺑﱠﻨَﺎ ﻻ َ ﻧﱠﺴِﯿﻨَﺎ أَوْ ﺗُﺆَاﺧِﺬْﻧَﺎ أَﺧْﻄَﺄ إِن ْﻧَﺎ رَﺑﱠﻨَﺎ و َﻻ َ ﺗَﺤْﻤِﻞْ ﻋَﻠَﯿْﻨَﺎ إِﺻْﺮًا ﻛَﻤَﺎ ﺣَﻤَﻠْﺘَﮫُ ﻋَﻠَﻰ َ ﻣِﻦ ﻗَﺒْﻠِﻨَﺎ رَﺑﱠﻨَﺎاﻟ و ﱠﺬِﯾﻦَﻻ َ ﺗُﺤَﻤِّﻠْﻨَﺎ ﻣَﺎ ﻻ َ طَﺎﻗَﺔَ ﻟَﻨَﺎ ﺑِﮫِ و َاﻋْﻒُ ﻋَﻨﱠﺎ و َاﻏْﻔِﺮْ ﻟَﻨَﺎ و َارْﺣَﻤْﻨَﺎ أَﻧﺖَ ﻣَﻮْﻻ َ ﻧَﺎ ﻓَﺎﻧﺼُﺮْﻧَﺎ ﻋَﻠَﻰ ﻘَﻮْمِاﻟْ اﻟْﻜَﺎﻓِﺮ ِﯾﻦَ ) ﺻدق اﻟﻠﮫ اﻟﻌظﯾم ﺳورة اﻟﺑﻘرة اﻷﯾﺔ (286) Dedication To whom he strives to bless comfort and welfare and never stints what he owns to push me in the success way who I taught me to promote life stairs wisely and patiently, to my dearest father To the Spring that never stops giving, to my mother who weaves my happiness with strings from her merciful heart To whose love flows in my veins, and my heart always remembers them, to my brothers ,sisters and my fiends To those who taught us letters of gold and words of jewel of the utmost and sweetest sentences in the whole knowledge. Who reworded to us their knowledge simply and from their thoughts made a lighthouse guides us through the knowledge and success path, To our honored teachers and professors. -

Quantum Mechanics Electromotive Force
Quantum Mechanics_Electromotive force . Electromotive force, also called emf[1] (denoted and measured in volts), is the voltage developed by any source of electrical energy such as a batteryor dynamo.[2] The word "force" in this case is not used to mean mechanical force, measured in newtons, but a potential, or energy per unit of charge, measured involts. In electromagnetic induction, emf can be defined around a closed loop as the electromagnetic workthat would be transferred to a unit of charge if it travels once around that loop.[3] (While the charge travels around the loop, it can simultaneously lose the energy via resistance into thermal energy.) For a time-varying magnetic flux impinging a loop, theElectric potential scalar field is not defined due to circulating electric vector field, but nevertheless an emf does work that can be measured as a virtual electric potential around that loop.[4] In a two-terminal device (such as an electrochemical cell or electromagnetic generator), the emf can be measured as the open-circuit potential difference across the two terminals. The potential difference thus created drives current flow if an external circuit is attached to the source of emf. When current flows, however, the potential difference across the terminals is no longer equal to the emf, but will be smaller because of the voltage drop within the device due to its internal resistance. Devices that can provide emf includeelectrochemical cells, thermoelectric devices, solar cells and photodiodes, electrical generators,transformers, and even Van de Graaff generators.[4][5] In nature, emf is generated whenever magnetic field fluctuations occur through a surface. -

Junction Analysis and Temperature Effects in Semi-Conductor
Junction analysis and temperature effects in semi-conductor heterojunctions by Naresh Tandan A thesis submitted to the Graduate Faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Electrical Engineering Montana State University © Copyright by Naresh Tandan (1974) Abstract: A new classification for semiconductor heterojunctions has been formulated by considering the different mutual positions of conduction-band and valence-band edges. To the nine different classes of semiconductor heterojunction thus obtained, effects of different work functions, different effective masses of carriers and types of semiconductors are incorporated in the classification. General expressions for the built-in voltages in thermal equilibrium have been obtained considering only nondegenerate semiconductors. Built-in voltage at the heterojunction is analyzed. The approximate distribution of carriers near the boundary plane of an abrupt n-p heterojunction in equilibrium is plotted. In the case of a p-n heterojunction, considering diffusion of impurities from one semiconductor to the other, a practical model is proposed and analyzed. The effect of temperature on built-in voltage leads to the conclusion that built-in voltage in a heterojunction can change its sign, in some cases twice, with the choice of an appropriate doping level. The total change of energy discontinuities (&ΔEc + ΔEv) with increasing temperature has been studied, and it is found that this change depends on an empirical constant and the 0°K Debye temperature of the two semiconductors. The total change of energy discontinuity can increase or decrease with the temperature. Intrinsic semiconductor-heterojunction devices are studied? band gaps of value less than 0.7 eV. -

CHAPTER 8: Diffusion
1 Chapter 8 CHAPTER 8: Diffusion Diffusion and ion implantation are the two key processes to introduce a controlled amount of dopants into semiconductors and to alter the conductivity type. Figure 8.1 compares these two techniques and the resulting dopant profiles. In the diffusion process, the dopant atoms are introduced from the gas phase of by using doped-oxide sources. The doping concentration decreases monotonically from the surface, and the in-depth distribution of the dopant is determined mainly by the temperature and diffusion time. Figure 8.1b reveals the ion implantation process, which will be discussed in Chapter 9. Generally speaking, diffusion and ion implantation complement each other. For instance, diffusion is used to form a deep junction, such as an n-tub in a CMOS device, while ion implantation is utilized to form a shallow junction, like a source / drain junction of a MOSFET. Boron is the most common p-type impurity in silicon, whereas arsenic and phosphorus are used extensively as n-type dopants. These three elements are highly soluble in silicon with solubilities exceeding 5 x 1020 atoms / cm3 in the diffusion temperature range (between 800oC and 1200oC). These dopants can be introduced via several means, including solid sources (BN for B, As2O3 for As, and P2O5 for P), liquid sources (BBr3, AsCl3, and POCl3), and gaseous sources (B2H6, AsH3, and PH3). Usually, the gaseous source is transported to the semiconductor surface by an inert gas (e.g. N2) and is then reduced at the surface. 2 Chapter 8 Figure 8.1: Comparison of (a) diffusion and (b) ion implantation for the selective introduction of dopants into a semiconductor substrate. -

Which Pnictogen Is Best?† Cite This: New J
NJC PAPER Pnictogen bonding with alkoxide cages: which pnictogen is best?† Cite this: New J. Chem., 2019, 43,14305 Henry J. Trubenstein, ‡ Shiva Moaven, ‡ Maythe Vega, Daniel K. Unruh and Anthony F. Cozzolino * Pnictogen bonding is beginning to emerge as a useful supramolecular interaction. The design strategies for these systems are still in the early stages of development and much attention has been focused on the lighter pnictogens. Pnictogen bond donors can have up to three independent sites for binding which can result in triple pnictogen bonding. This has been observed in the self-assembly of antimony alkoxide cages, but not with the lighter congeners. This work reports structural characterization of an analogous arsenic alkoxide cage that engages in a single pnictogen bond and synthetic explorations of Received 14th July 2019, the bismuth congener. DFT calculations are used to evaluate the differences between the structures. Accepted 13th August 2019 Ultimately the partial charge on the pnictogen and the energy of the pnictogen lone pair dictate the DOI: 10.1039/c9nj03648b strength, orientation and number of pnictogen bonds that these cages form. Antimony cages strike the best balance between strength and directionality, allowing them to achieve triple pnictogen bonding rsc.li/njc where the other congeners do not. Introduction or bismuth.15–23 Recently, antimony centred PnBs have been purposefully designed into molecules to actively direct the self- A pnictogen bond (PnB), in analogy to a halogen or chalcogen assembly of reversed bilayer vesicles,24 enable anion binding bond (XB/HaB or ChB), is ‘‘the net attractive interaction between with applications in sensing and transport,25–28 self-assembly an electrophilic region associated with a [pnictogen] atom in a complex architectures through triple pnictogen bonding29 and molecular entity [the PnB donor] and a nucleophilic region in allow for supramolecular catalysis.30 These applications rely on another, or the same, molecular entity [the PnB acceptor].’’1 the predictable formation of PnBs. -

Introduction to Semiconductor
Introduction to semiconductor Semiconductors: A semiconductor material is one whose electrical properties lie in between those of insulators and good conductors. Examples are: germanium and silicon. In terms of energy bands, semiconductors can be defined as those materials which have almost an empty conduction band and almost filled valence band with a very narrow energy gap (of the order of 1 eV) separating the two. Types of Semiconductors: Semiconductor may be classified as under: a. Intrinsic Semiconductors An intrinsic semiconductor is one which is made of the semiconductor material in its extremely pure form. Examples of such semiconductors are: pure germanium and silicon which have forbidden energy gaps of 0.72 eV and 1.1 eV respectively. The energy gap is so small that even at ordinary room temperature; there are many electrons which possess sufficient energy to jump across the small energy gap between the valence and the conduction bands. 1 Alternatively, an intrinsic semiconductor may be defined as one in which the number of conduction electrons is equal to the number of holes. Schematic energy band diagram of an intrinsic semiconductor at room temperature is shown in Fig. below. b. Extrinsic Semiconductors: Those intrinsic semiconductors to which some suitable impurity or doping agent or doping has been added in extremely small amounts (about 1 part in 108) are called extrinsic or impurity semiconductors. Depending on the type of doping material used, extrinsic semiconductors can be sub-divided into two classes: (i) N-type semiconductors and (ii) P-type semiconductors. 2 (i) N-type Extrinsic Semiconductor: This type of semiconductor is obtained when a pentavalent material like antimonty (Sb) is added to pure germanium crystal. -

Chapter 4: Bonding in Solids and Electronic Properties
Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal – an electron gas. •regards a metal as a box in which electrons are free to move. •assumes nuclei stay fixed on their lattice sites surrounded by core electrons, while the valence electrons move freely through the solid. •Ignoring the core electrons, one can treat the outer electrons with a quantum mechanical description. •Taking just one electron, the problem is reduced to a particle in a box. Electron is confined to a line of length a. Schrodinger equation 2 2 2 d d 2me E 2 (E V ) 2 2 2me dx dx Electron is not allowed outside the box, so the potential is ∞ outside the box. Energy is quantized, with quantum numbers n. 2 2 2 n2h2 h2 n n n In 1d: E In 3d: E a b c 2 8m a2 b2 c2 8mea e 1 2 2 2 2 Each set of quantum numbers n , n , h n n n a b a b c E 2 2 2 and nc will give rise to an energy level. 8me a b c •In three dimensions, there are multiple combination of energy levels that will give the same energy, whereas in one-dimension n and –n are equal in energy. 2 2 2 2 2 2 na/a nb/b nc/c na /a + nb /b + nc /c 6 6 6 108 The number of states with the 2 2 10 108 same energy is known as the degeneracy. 2 10 2 108 10 2 2 108 When dealing with a crystal with ~1020 atoms, it becomes difficult to work out all the possible combinations. -
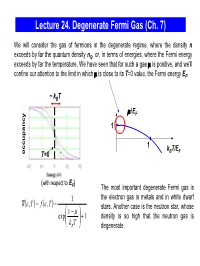
Lecture 24. Degenerate Fermi Gas (Ch
Lecture 24. Degenerate Fermi Gas (Ch. 7) We will consider the gas of fermions in the degenerate regime, where the density n exceeds by far the quantum density nQ, or, in terms of energies, where the Fermi energy exceeds by far the temperature. We have seen that for such a gas μ is positive, and we’ll confine our attention to the limit in which μ is close to its T=0 value, the Fermi energy EF. ~ kBT μ/EF 1 1 kBT/EF occupancy T=0 (with respect to E ) F The most important degenerate Fermi gas is 1 the electron gas in metals and in white dwarf nε()(),, T= f ε T = stars. Another case is the neutron star, whose ε⎛ − μ⎞ exp⎜ ⎟ +1 density is so high that the neutron gas is ⎝kB T⎠ degenerate. Degenerate Fermi Gas in Metals empty states ε We consider the mobile electrons in the conduction EF conduction band which can participate in the charge transport. The band energy is measured from the bottom of the conduction 0 band. When the metal atoms are brought together, valence their outer electrons break away and can move freely band through the solid. In good metals with the concentration ~ 1 electron/ion, the density of electrons in the electron states electron states conduction band n ~ 1 electron per (0.2 nm)3 ~ 1029 in an isolated in metal electrons/m3 . atom The electrons are prevented from escaping from the metal by the net Coulomb attraction to the positive ions; the energy required for an electron to escape (the work function) is typically a few eV. -

Chapter 13 Ideal Fermi
Chapter 13 Ideal Fermi gas The properties of an ideal Fermi gas are strongly determined by the Pauli principle. We shall consider the limit: k T µ,βµ 1, B � � which defines the degenerate Fermi gas. In this limit, the quantum mechanical nature of the system becomes especially important, and the system has little to do with the classical ideal gas. Since this chapter is devoted to fermions, we shall omit in the following the subscript ( ) that we used for the fermionic statistical quantities in the previous chapter. − 13.1 Equation of state Consider a gas ofN non-interacting fermions, e.g., electrons, whose one-particle wave- functionsϕ r(�r) are plane-waves. In this case, a complete set of quantum numbersr is given, for instance, by the three cartesian components of the wave vector �k and thez spin projectionm s of an electron: r (k , k , k , m ). ≡ x y z s Spin-independent Hamiltonians. We will consider only spin independent Hamiltonian operator of the type ˆ 3 H= �k ck† ck + d r V(r)c r†cr , �k � where thefirst and the second terms are respectively the kinetic and th potential energy. The summation over the statesr (whenever it has to be performed) can then be reduced to the summation over states with different wavevectork(p=¯hk): ... (2s + 1) ..., ⇒ r � �k where the summation over the spin quantum numberm s = s, s+1, . , s has been taken into account by the prefactor (2s + 1). − − 159 160 CHAPTER 13. IDEAL FERMI GAS Wavefunctions in a box. We as- sume that the electrons are in a vol- ume defined by a cube with sidesL x, Ly,L z and volumeV=L xLyLz. -

Donor Qubits in Silicon
04/09/2015 Experiments with spin qubits in siliconDonor and diamond qubits in silicon Gavin W Morley University of Warwick Gavin W Morley, Spin qubits in silicon and diamond, QuICC Warwick, August 2015 Experiments with spin qubits in silicon and diamond: overview • Lecture 1 – Magnetic resonance – Silicon • Lecture 2 – Silicon (cont.) Lab tours • Lecture 3 Friday morning – Diamond Gavin W Morley, Spin qubits in silicon and diamond, QuICC Warwick, August 2015 1 04/09/2015 Experiments with spin qubits in silicon and diamond: overview • Lecture 1 – Magnetic resonance 1. Prepare (spin Hamiltonian) 2. Control (electromagnetic pulses) 3. Measure (spin state readout) – Silicon Gavin W Morley, Spin qubits in silicon and diamond, QuICC Warwick, August 2015 – Magnetic resonance 1. Prepare (spin Hamiltonian) 2. Control (electromagnetic pulses) 3. Measure (spin state readout) Gavin W Morley, Spin qubits in silicon and diamond, QuICC Warwick, August 2015 2 04/09/2015 Magnetic resonance: prepare - put a spin ½ into a magnetic field H Ŝ Hamiltonian = ωS z with energy, ħ ωS = g µB B0 for g-factor, g ~ 2, Bohr magneton µB = e ħ/2me, electron spin S = ½ Energy m = ½ of a s spin Magnetic field, B0 ms = -½ Gavin W Morley, Spin qubits in silicon and diamond, QuICC Warwick, August 2015 Magnetic resonance: prepare - put a spin ½ into a magnetic field Larmor precession at angular frequency ωS Gavin W Morley, Spin qubits in silicon and diamond, QuICC Warwick, August 2015 3 04/09/2015 Magnetic resonance: prepare - put a spin ½ into a magnetic field Larmor precession -

Donor-Acceptor Methods for Band Gap Reduction in Conjugated Polymers: the Role of Electron Rich Donor Heterocycles
DONOR-ACCEPTOR METHODS FOR BAND GAP REDUCTION IN CONJUGATED POLYMERS: THE ROLE OF ELECTRON RICH DONOR HETEROCYCLES By CHRISTOPHER A. THOMAS A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2002 Copyright 2002 by Christopher A. Thomas All rights reserved. ACKNOWLEDGMENTS I thank my parents, Nancy and Larry, for their continuous support and attempts to understand and encourage me during what has been simultaneously the most enjoyable and most stressful part of my life. They made this document possible by encouraging and par- ticipating in experiences that ensured I would have the backgound and interest in trying to figure out how the world works. The decision about whom to work for in graduate school is one of the events I have agonized the most about in my life. As promised, the graduate advisor-student relationship is a complicated entity consisting of advisor, boss, counselor and friend that can change its active role without warning. Despite this, I am positive that there is no other person that I would rather have worked with during this process. I especially appreciate being given an unusual amount of decision making and direction setting power in the projects I was involved with and the freedom to explore aspects of science that interested me even when they did not overlap cleanly with Professor Reynolds’ research interests or funding. For their major contributions to my enjoyment and interest in chemistry, I thank Joe Carolan for his contagious enthusiasm, and Joel Galanda, who immensely affected the three years of my life he was involved with my chemistry and physical science education.