False Claims Act Alert
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FY 2014 Management Challenges
U.S. Department of Education Office of Inspector General FY 2014 Management Challenges November 2013 Office of Inspector General Kathleen S. Tighe Inspector General November 2013 This report is in the public domain. Authorization to reproduce it in whole or in part is granted. While permission to reprint this publication is not necessary, the citation should be: U.S. Department of Education, Office of Inspector General, FY 2014 Management Challenges. Please Note: The Inspector General’s FY 2014 Management Challenges is available on the ED OIG Web site at http://www2.ed.gov/about/offices/list/oig/reports.html. UNITED STATES DEPARTMENT OF EDUCATION The Inspector General November 12, 2013 MEMORANDUM TO: The Honorable Arne Duncan Secretary of Education FROM: Kathleen S. Tighe Inspector General SUBJECT: Management Challenges for Fiscal Year 2014 The Reports Consolidation Act of 2000 requires the U.S. Department of Education (Department), Office of Inspector General to identify and report annually on the most serious management challenges the Department faces. The Government Performance and Results Modernization Act of 2010 requires the Department to include in its agency performance plan information on its planned actions, including performance goals, indicators, and milestones, to address these challenges. To identify management challenges, we routinely examine past audit, inspection, and investigative work, as well as issued reports where corrective actions have yet to be taken; assess ongoing audit, inspection, and investigative work to identify significant vulnerabilities; and analyze new programs and activities that could post significant challenges because of their breadth and complexity. Last year, we presented four management challenges: improper payments, information technology security, oversight and monitoring, and data quality and reporting. -

Comments from the Legal Aid Community to the Department of Education Re
Comments from the Legal Aid Community to the Department of Education re: Proposed Regulations on Program Integrity and Improvement: State Authorization of Distance Education Programs Docket ID ED-2016-OPE-0050 August 24, 2016 Comments submitted on behalf of: East Bay Community Law Center Empire Justice Center Housing and Economic Rights Advocates National Consumer Law Center (on behalf of its low-income clients) LAF (formerly Legal Assistance Foundation of Metropolitan Chicago) Legal Aid Foundation of Los Angeles Legal Aid Society of San Diego, Inc. Legal Services NYC New York Legal Assistance Group Project on Predatory Student Lending of the Legal Services Center of Harvard Law School Public Law Center Margaret Reiter in her individual capacity Introduction These comments, submitted on behalf of organizations across the country that provide free legal assistance to low-income student loan borrowers, address the Department’s proposed regulations regarding the state authorization of distance education programs.1 Our comments are informed by our work as legal aid practitioners. We strive to meet the legal needs of individuals 1 81 Fed. Reg. 48598 (proposed July 25, 2016). 1 and families with limited economic means, who otherwise would be without professional legal assistance. Margaret Reiter also joins in these comments in her individual capacity, not as a representative of any organization or agency. She was a consumer investigator with the Los Angeles County Consumer Affairs Department for four years and worked for 20 years as a consumer prosecutor with the California Attorney General’s Consumer Law Section. She investigated or prosecuted businesses engaged in many types of misrepresentations and unlawful business practices, including postsecondary for-profit schools. -

State Inaction: Gaps in State Oversight of For-Profit Higher
STATE INACTION GAPS IN STATE OVERSIGHT OF FOR-PROFIT HIGHER EDUCATION NCLC® NATIONAL CONSUMER December 2011 LAW CENTER® © Copyright 2011, National Consumer Law Center, Inc. All rights reserved. ABOUT THE AUTHORS Deanne Loonin is a staff attorney at the National Consumer Law Center (NCLC) and the Director of NCLC’s Student Loan Borrower Assistance Project. She was formerly a legal services attorney in Los Angeles. She is the author of numerous publications and reports, including NCLC publications Student Loan Law and Surviving Debt. Jillian McLaughlin is a research assistant at NCLC. She graduated from Kalamazoo College with a degree in political science. ACKNOWLEDGMENTS This report is a release of the National Consumer Law Center’s Student Loan Borrower Assis- tance Project [www.studentloanborrowerassistance.org]. The authors thank NCLC colleagues Carolyn Carter, Jan Kruse, and Persis Yu for valuable comments and assistance. We also thank Allen Agnitti, Laura Kirshner, and Kurt Terwilliger for research assistance. The findings and conclusions presented in this report are those of the authors alone. NCLC® ABOUT THE NATIONAL CONSUMER LAW CENTER The National Consumer Law Center®, a nonprofit corporation founded in 1969, assists NATIONAL consumers, advocates, and public policy makers nationwide on consumer law issues. CONSUMER NCLC works toward the goal of consumer justice and fair treatment, particularly for those whose poverty renders them powerless to demand accountability from the economic LAW marketplace. NCLC has provided model language and testimony on numerous consumer CENTER law issues before federal and state policy makers. NCLC publishes an 18-volume series ® of treatises on consumer law, and a number of publications for consumers. -

Government Investigations/Lawsuits of For-Profit Schools
NCLC® ensuring NATIONAL CONSUMER educational LAW integrity CENTER ® 10 STEPS TO IMPROVE STATE OVERSIGHT OF FOR-PROFIT SCHOOLS © Copyright 2014, National Consumer Law Center, Inc. GOVERNMENT INVESTIGATIONS AND LAWSUITS INVOLVING FOR-PROFIT SCHOOLS1 (2004 – MAY 2014) ©2014 National Consumer Law Center www.nclc.org Ensuring Educational Integrity, In Their Own Words 5 1 Note: Chart is organized alphabetically by government agency. SCHOOLS OUTCOME OR OFFER ONLINE/ SCHOOLS/ GOVERNMENT INVESTIGATION PENDING ACCREDITOR DISTANCE OWNERS AGENCY OR LAWSUIT? DATE ALLEGATIONS OR ISSUES (AS OF JUNE 1, 2014) (IF ANY) PROGRAMS? CREDENTIALS OFFERED Corinthian AGs from AR, Multi-state 1/2014 Organizational information; tuition, loan Pending National— Everest Univ. Certificates, Associate, Colleges, Inc.2 AZ, CO, CT, HI, Investigation and scholarship information; lead generation Everest Colleges (ACCSC); Online; Everest Bachelor’s and Master’s ID, IA, KY, MO, activities; enrollment qualifications Everest Univ. Online (ACICS); College Phoenix Degrees NC, NE, NM, for students; complaints; accreditation; Wyotech (ACCSC); OR, PA, TN, WA completion and placement statistics; graduate Regional— certification and licensing results; student Everest College Phoenix (HLC); lending activities. Heald (WASC Senior College and University Commission)3 ITT Educational AGS from AR, Multi-state 1/2014 Marketing and advertising, recruitment, Pending National (ACICS)5 Yes Associate, Bachelor’s and Services, Inc.4 AZ, CT, ID, IA, Investigation financial aid, academic advising, career Master’s Degrees KY, MO, NE, NC, services, admissions, licensure exam pass OR, PA, TN and rates, accreditation, student retention, WA graduation rates and job placement rates. Career Education AGs from AR, Multi-state 1/2014 Student-recruitment practices, graduate Pending National— Yes Certificate, Associate, Corp.6 AZ, CT, ID, IA, Investigation employment statistics, graduate employment Sanford-Brown. -

For-Profit Education and the False Claims Act
For-Profit Education and the False Claims Act Written by Nick Sanders The United States government has intervened in a qui tam suit brought under the False Claims Act against ATI Enterprises, Inc. (which does business as ATI Technical Training Center, ATI Career Training Center and ATI Career Training) and which “operates career college campuses in Texas, Florida, Oklahoma and New Mexico,” according to this announcement by the Department of Justice. The complaint against ATI alleged that— ATI Enterprises knowingly misrepresented its job placement statistics to the Texas Workforce Commission in order to maintain its state licensure, and therefore its eligibility for federal financial aid under Title IV of the Higher Education Act of 1965, as amended. On Aug. 9, 2011, the Texas Workforce Commission revoked licenses for several of ATI’s programs at the three campuses after a third party audit of ATI’s reported placement statistics. The DOJ press release provided some more details. It reported— … ATI employees at the three campuses knowingly enrolled students who were ineligible because they did not have high school diplomas or recognized equivalents; falsified high school diplomas, including five Dallas Independent School District diplomas for students who later defaulted on their federal student loans; fraudulently kept students enrolled even though they should have been dropped because they had poor grades or attendance; and made knowing misrepresentations to students about their future employability. [Allegedly.] The alleged misrepresentations included telling students that a criminal record would not prevent them from getting jobs in their fields of study, quoting higher salaries than the students would be likely to earn and reporting inflated job placement statistics both to the students and the 1 / 2 For-Profit Education and the False Claims Act Written by Nick Sanders Texas Workforce Commission. -

For-Profit-Report.Pdf
ENSURING EDUCATIONAL INTEGRITY 10 STEPS TO IMPROVE STATE OVERSIGHT OF FOR-PROFIT SCHOOLS NCLC® NATIONAL CONSUMER June 2014 LAW CENTER® © Copyright 2014, National Consumer Law Center, Inc. All rights reserved. ABOUT THE AUTHOR Robyn Smith is Of Counsel for the National Consumer Law Center, where she concentrates on student loan and proprietary school issues. She also focuses on these issues as a staff attorney at the Legal Aid Foundation of Los Angeles. From 2001 to 2010, she worked in the Consumer Law Section of the California Attorney General’s office, where she investigated and prosecuted businesses engaged in deceptive business practices, including for-profit colleges. Prior to joining the AG’s office, Smith represented low-income consumers in a wide range of consumer law matters as the Directing Attorney of the Consumer Law Project at Public Counsel in Los Angeles and as the Managing Attorney of the Windward Branch of the Legal Aid Society of Hawaii on the island of Oahu. She received her J.D. from University of Southern California. ACKNOWLEDGMENTS The author thanks NCLC colleagues Deanne Loonin, Carolyn Carter, and Jan Kruse for valuable comments and assistance. She also thanks Emily Green Kaplan, Juala Smythe, and Shirlron Williams for research assistance. The findings and conclusions presented in this report are those of the author alone. NCLC’s Student Loan Borrower Assistance Project provides information about student loan rights and responsibilities for borrowers and advocates. We also seek to increase public understanding of student lending issues and to identify policy solutions to promote access to education, lessen student debt burdens, and make loan repayment more manageable. -

List of For-Profit Universities and Colleges
List of for-profit universities and colleges This is a list of for-profit institutions of higher education. Contents In the United States Closed Outside the United States Distance education (online) See also References In the United States Academy of Art University – San Francisco, California American Career College – Los Angeles, California American College of Education – online American InterContinental University – more than 90% online, a subsidiary of Career Education Corporation American Military University – online, a division of American Public University System American National University – distance education and multiple locations in Virginia, Ohio, Kentucky, Indiana, and West Virginia; not to be confused with American University American Public University – online American Sentinel University – online Antilles College of Health – Puerto Rico Antonelli College – multiple locations Arizona Summit Law School – a subsidiary of InfiLaw System ASA College – campuses in Brooklyn, midtown Manhattan, and Miami Ashford University – Clinton, Iowa (campus closed in 2016), a subsidiary of Bridgepoint Education, includes Forbes School of Business Aspen University – Denver, Colorado Atenas College – multiple locations, Puerto Rico Bay State College – Boston, Massachusetts Beckfield College – a subsidiary of Quad Partners Berkeley College – New York and New Jersey; not to be confused with University of California, Berkeley, Berklee School of Music or the Berkeley College at Yale University Blair College – Colorado Springs, Colorado - Now Everest -

FY 2016 Management Challenges for U.S. Department of Education
FY 2016 Management Challenges October 2015 U.S. Department of Education Office of Inspector General Office of Inspector General Kathleen S. Tighe Inspector General October 2015 This report is in the public domain. Authorization to reproduce it in whole or in part is granted. While permission to reprint this publication is not necessary, the citation should be: U.S. Department of Education, Office of Inspector General, FY 2016 Management Challenges. Please Note: The Inspector General’s FY 2016 Management Challenges is available on the ED OIG Web site at http://www2.ed.gov/about/offices/list/oig/ reports.html. Cover image by Jannis Tobias Werner/Shutterstock.com. All other images used under license from Shutterstock.com. UNITED STATES DEPARTMENT OF EDUCATION OFFICE OF INSPECTOR GENERAL The Inspector General October 9, 2015 TO: The Honorable Arne Duncan Secretary of Education FROM: Kathleen S. Tighe Inspector General SUBJECT: Management Challenges for Fiscal Year 2016 The Reports Consolidation Act of 2000 requires the U.S. Department of Education (Department), Office of Inspector General to identify and report annually on the most serious management challenges the Department faces. The Government Performance and Results Modernization Act of 2010 requires the Department to include in its agency performance plan information on its planned actions, including performance goals, indicators, and milestones, to address these challenges. To identify management challenges, we routinely examine past audit, inspection, and investigative work, as well as issued reports where corrective actions have yet to be taken; assess ongoing audit, inspection, and investigative work to identify significant vulnerabilities; and analyze new programs and activities that could post significant challenges because of their breadth and complexity. -

South Texas Vocational Technical Institute 2021 School Catalog Version 1 Effective: January 4, 2021
South Texas Vocational Technical Institute 2021 School Catalog Version 1 Effective: January 4, 2021 Campus Locations South Texas Vocational Technical Institute – Weslaco Campus 1600 N. Westgate Drive, Suite 400 Weslaco, Texas 78599 Phone: 956-969-1564 Fax: 956-969-1887 South Texas Vocational Technical Institute – Brownsville Campus (a branch of the Weslaco Campus) 1900 N. Expressway, Suite O Satellite Facility – CDL Range Brownsville, Texas 78521 2800 Robindale Road Phone: 956-554-3515 Brownsville, TX 78521 Fax: 956-554-3542 South Texas Vocational Technical Institute – McAllen Campus (a branch of the Platt College ‐ Tulsa Campus) 1800 S. Main Street, Suite 500 Satellite Facility – CDL Range McAllen, TX 78503 901 E Military Highway Phone: 956-631-1107 Pharr, TX 78503 Fax 956-630-1650 South Texas Vocational Technical Institute – Corpus Christi Campus (a branch of the Platt College ‐ Tulsa Campus) 2000 South Padre Island Drive Corpus Christi, TX 78416 Phone: 361-232-5057 Fax: 361-851-5051 South Texas Vocational Technical Institute – San Antonio, TX (a branch of Platt College – Tulsa Campus) 734 S.E. Military Drive Extension Building Satellite Facility – CDL Range San Antonio, TX 78214 6714 S Flores Street 9333 Southwest Loop 410 Phone: 210-782-8000 San Antonio, TX 78214 San Antonio, TX 78242 Fax: 210-921-0513 www.stvt.edu Page 1 of 163 Table of Contents Campus Locations ..................................................................................................................................... 1 Table of Contents ...................................................................................................................................... -
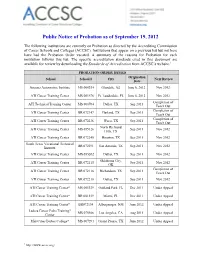
Public Notice of Probation As of September 19, 2012
ACCSC Summary of Grounds for Probation Action Updated September 19, 2012 Page 1 of 9 Public Notice of Probation as of September 19, 2012 The following institutions are currently on Probation as directed by the Accrediting Commission of Career Schools and Colleges (ACCSC). Institutions that appear on a previous list but not here have had the Probation Order vacated. A summary of the reasons for Probation for each institution follows this list. The specific accreditation standards cited in this document are available for review by downloading the Standards of Accreditation from ACCSC’s website.1 PROBATION ORDER ISSUED Origination School School # City Next Review Date Arizona Automotive Institute MS 000334 Glendale, AZ June 6, 2012 Nov 2012 ATI Career Training Center MS 001576 Ft. Lauderdale, FL June 6, 2012 Nov 2012 Completion of ATI Technical Training Center MS 000704 Dallas, TX Sep 2011 Teach Out Completion of ATI Career Training Center BR 072147 Garland, TX Sep 2011 Teach Out Completion of ATI Career Training Center BR 072236 Waco, TX Sep 2011 Teach Out North Richland ATI Career Training Center MS 055126 Sep 2011 Nov 2012 Hills, TX ATI Career Training Center BR 072240 Houston, TX Sep 2011 Nov 2012 South Texas Vocational Technical BR 072291 San Antonio, TX Sep 2011 Nov 2012 Institute ATI Career Training Center MS 055202 Dallas, TX Sep 2011 Nov 2012 Oklahoma City, ATI Career Training Center BR 072115 Sep 2011 Nov 2012 OK Completion of ATI Career Training Center BR 072116 Richardson, TX Sep 2011 Teach Out ATI Career Training Center BR 072210 -

Department of Education OIG SAR 67 (4-1-13 Through 9
U.S. Department of Education Office of Inspector General Semiannual Report to Congress, No. 67 April 1, 2013–September 30, 2013 Office of Inspector General Kathleen S. Tighe Inspector General December 2013 This report is in the public domain. Authorization to reproduce it in whole or in part is granted. While permission to reprint this publication is not necessary, the citation should be: U.S. Department of Education, Office of Inspector General, Semiannual Report to Congress, No. 67. Please Note: The Inspector General’s Semiannual Report to Congress, No. 67 is available on the ED OIG Web site at http://www2.ed.gov/about/offices/list/oig/sarpages.html. Message to Congress On behalf of the U.S. Department of Education (Department) Office of Inspector General (OIG), I present this Semiannual Report on the activities and accomplishments of this office from April 1, 2013, through September 30, 2013. The audits, investigations, and related work highlighted in the report are products of our continuing commitment to promoting accountability, efficiency, and effectiveness in our oversight of the Department’s programs and operations. Over the last 6 months, we closed 74 investigations involving fraud or corruption related to the Department’s programs and operations, securing more than $44.8 million in settlements, fines, restitutions, recoveries, and savings. In addition, as a result of our investigative work, criminal actions were taken against a number of individuals, including school officials—people who cheated the students they were in positions to serve. We also issued 15 audit-related reports, making recommendations to improve program operations. For example and as highlighted in this report: A 2012 OIG audit identified a possible conflict involving the Alabama State Department of Education’s Director of Federal Programs’ participation in the selection process that awarded $24 million to three local educational agencies that listed her husband’s employer as a contractor. -

For Profit Higher Education: the Failure to Safeguard the Federal
United States Senate HEALTH, EDUCATION, LABOR AND PENSIONS COMMITTEE For Profi t Higher Education: The Failure to Safeguard the Federal Investment and Ensure Student Success Majority Committee Staff Report and Accompanying Minority Committee Staff Views July 30, 2012 Contents Executive Summary ........................................................................................................... 1 Introduction ...................................................................................................................... 12 Institutions Examined ...................................................................................................... 20 Publicly Traded Companies ..................................................................................................... 20 Private Equity Owned Companies ........................................................................................... 22 Closely Held Corporations ....................................................................................................... 23 The Federal Investment and the Changing Sector ........................................................ 24 Increasing Federal Investment .................................................................................................. 24 Increasing Reliance on Federal Dollars .................................................................................... 24 Pell Grant Funds ......................................................................................................................