Online Journal of Animal and Feed Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Analysis of the Afar-Somali Conflict in Ethiopia and Djibouti
Regional Dynamics of Inter-ethnic Conflicts in the Horn of Africa: An Analysis of the Afar-Somali Conflict in Ethiopia and Djibouti DISSERTATION ZUR ERLANGUNG DER GRADES DES DOKTORS DER PHILOSOPHIE DER UNIVERSTÄT HAMBURG VORGELEGT VON YASIN MOHAMMED YASIN from Assab, Ethiopia HAMBURG 2010 ii Regional Dynamics of Inter-ethnic Conflicts in the Horn of Africa: An Analysis of the Afar-Somali Conflict in Ethiopia and Djibouti by Yasin Mohammed Yasin Submitted in partial fulfilment of the requirements for the degree PHILOSOPHIAE DOCTOR (POLITICAL SCIENCE) in the FACULITY OF BUSINESS, ECONOMICS AND SOCIAL SCIENCES at the UNIVERSITY OF HAMBURG Supervisors Prof. Dr. Cord Jakobeit Prof. Dr. Rainer Tetzlaff HAMBURG 15 December 2010 iii Acknowledgments First and foremost, I would like to thank my doctoral fathers Prof. Dr. Cord Jakobeit and Prof. Dr. Rainer Tetzlaff for their critical comments and kindly encouragement that made it possible for me to complete this PhD project. Particularly, Prof. Jakobeit’s invaluable assistance whenever I needed and his academic follow-up enabled me to carry out the work successfully. I therefore ask Prof. Dr. Cord Jakobeit to accept my sincere thanks. I am also grateful to Prof. Dr. Klaus Mummenhoff and the association, Verein zur Förderung äthiopischer Schüler und Studenten e. V., Osnabruck , for the enthusiastic morale and financial support offered to me in my stay in Hamburg as well as during routine travels between Addis and Hamburg. I also owe much to Dr. Wolbert Smidt for his friendly and academic guidance throughout the research and writing of this dissertation. Special thanks are reserved to the Department of Social Sciences at the University of Hamburg and the German Institute for Global and Area Studies (GIGA) that provided me comfortable environment during my research work in Hamburg. -
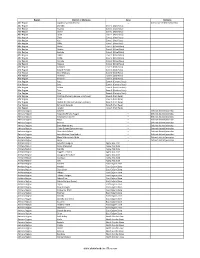
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

ETHIOPIA Humanitarian Access Situation Report June – July 2019
ETHIOPIA Humanitarian Access Situation Report June – July 2019 This report is produced by OCHA Ethiopia in collaboration with humanitarian partners. It covers the period June - July 2019. The next report will be issued around September - October 2019. OVERVIEW IUS • In June - July, Ethiopia experienced an at- TIGRAY 276 Access incidents reported tempted government overthrow in Amhara, Western socio-political unrest in Sidama (SNNPR), North Gondar Wag Hamra Central Gondar and a rise in security incidents in Southwest- Zone 4 (Fantana Rasu) AFAR ern Oromia and Gambella. The quality of ac- Zone 1 (Awsi Rasu) cess declined, limiting assistance to people AMHARA No. o incidents by one South Wello Metekel in need, against a backdrop of massive gov- Oromia East Gojam BENISHANGUL Zone 5 (Hari Rasu) 4 13 35 49 AsosaGUMUZ Siti ernment-led returns of IDP to areas of origin. Zone 3 (Gabi Rasu) North Shewa(O) North Shewa(A) Kemashi Dire Dawa urban West Wellega East Wellega DIRE DAWA West Shewa Fafan • Hostilities between Ethiopian Defense Forc- ADDIS ABABA Kelem Wellega East Hararge Finfine Special West Hararge es (EDF) and Unidentified Armed Groups Buno Bedele East Shewa Etang Special Ilu Aba Bora Jarar OROMIA Erer (UAGs) as well as inter-ethnic, remained the GAMBELA Jimma Agnewak main access obstacle, with 197 incidents Doolo Nogob West Arsi SOMALI (out of 276), mostly in Southwestern Oromia SNNP Sidama Bale Korahe (110). The Wellegas, West Guji (Oromia), and Gedeo Shabelle Gambella, were the most insecure areas for Segen Area P. West Guji Guji aid workers. Liban Borena • In June, conflict in the Wellegas scaled up, Daawa with explosive devices attacks causing ci- Source: Access Incidents database vilian casualties in urban centres. -

List of Zones of Ethiopia
Region Zone Addis Ababa Addis Ababa Afar Region Administrative Zone 1 (a.k.a. Awsi Rasu) Afar Region Administrative Zone 2 (a.k.a. Kilbet Rasu) Afar Region Administrative Zone 3 (a.k.a. Gabi Rasu) Afar Region Administrative Zone 4 (a.k.a. Fantena Rasu) Afar Region Administrative Zone 5 (a.k.a. Hari Rasu) Afar Region Argobba (special woreda) Amhara Region Agew Awi Amhara Region East Gojjam Amhara Region North Gondar Amhara Region North Shewa Amhara Region North Wollo Amhara Region Oromia Amhara Region South Gondar Amhara Region South Wollo Amhara Region Wag Hemra Amhara Region West Gojjam Amhara Region Bahir Dar (special zone) Benishangul-Gumuz Region Asosa Benishangul-Gumuz Region Kamashi Benishangul-Gumuz Region Metekel Dire Dawa Dire Dawa Gambela Region Anuak Gambela Region Mezhenger Gambela Region Nuer Harari Region Harari Oromia Region Arsi Oromia Region Bale Oromia Region Borena Oromia Region East Hararghe Oromia Region East Shewa Oromia Region East Welega Oromia Region Guji Oromia Region Horo Gudru Welega Oromia Region Illubabor Oromia Region Jimma Oromia Region Kelem Welega Oromia Region North Shewa Oromia Region South West Shewa Oromia Region West Arsi Oromia Region West Hararghe Oromia Region West Shewa Oromia Region West Welega Oromia Region Adama (special zone) Oromia Region Jimma (special zone) Oromia Region Oromia-Finfinne (special zone) www.downloadexcelfiles.com Somali Region Afder Somali Region Degehabur Somali Region Fiq Somali Region Gode Somali Region Jijiga Somali Region Korahe Somali Region Liben Somali Region Shinile -

Ethiopia COI Compilation
BEREICH | EVENTL. ABTEILUNG | WWW.ROTESKREUZ.AT ACCORD - Austrian Centre for Country of Origin & Asylum Research and Documentation Ethiopia: COI Compilation November 2019 This report serves the specific purpose of collating legally relevant information on conditions in countries of origin pertinent to the assessment of claims for asylum. It is not intended to be a general report on human rights conditions. The report is prepared within a specified time frame on the basis of publicly available documents as well as information provided by experts. All sources are cited and fully referenced. This report is not, and does not purport to be, either exhaustive with regard to conditions in the country surveyed, or conclusive as to the merits of any particular claim to refugee status or asylum. Every effort has been made to compile information from reliable sources; users should refer to the full text of documents cited and assess the credibility, relevance and timeliness of source material with reference to the specific research concerns arising from individual applications. © Austrian Red Cross/ACCORD An electronic version of this report is available on www.ecoi.net. Austrian Red Cross/ACCORD Wiedner Hauptstraße 32 A- 1040 Vienna, Austria Phone: +43 1 58 900 – 582 E-Mail: [email protected] Web: http://www.redcross.at/accord This report was commissioned by the United Nations High Commissioner for Refugees (UNHCR), Division of International Protection. UNHCR is not responsible for, nor does it endorse, its content. TABLE OF CONTENTS List of abbreviations ........................................................................................................................ 4 1 Background information ......................................................................................................... 6 1.1 Geographical information .................................................................................................... 6 1.1.1 Map of Ethiopia ........................................................................................................... -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

ETHIOPIA Humanitarian Access Situation Report October - December 2019
ETHIOPIA Humanitarian Access Situation Report October - December 2019 This report is produced by OCHA Ethiopia in collaboration with humanitarian partners. It covers the period October - December 2019. The next report will be issued on March 2020. OVERVIEW ! ! ! ! ! ! ! ! ! ! ! Key figures ! ! ! ! ! ! ! ! ! ! ! ! ! • The operational environment to relief opera- ! ! ! ! ! ! ! ! ! Tigray ! ! ! ! ! Access incidents reported ! ! 259 ! ! ! ! ! ! Western ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !! ! ! ! ! ! ! tions in Ethiopia remained permissive through ! ! ! ! ! ! ! ! ! ! ! 1 Aid worker killed ! ! ! ! ! ! ! ! North! Gondar ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! the reporting period. ! ! ! ! ! ! Aid workers injured ! ! ! ! ! ! 2 ! ! ! ! ! ! ! ! ! ! ! ! ! Wag! Hamra ! ! ! ! ! ! ! ! ! ! ! Central Gondar ! Southern ! ! ! ! ! ! West Gondar ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Zone 4 (Fantana Rasu) ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Afar ! ! ! ! ! North Wello! ! No. of incidents by woreda ! ! ! ! • Localized armed confrontations, ethnic vio- ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! South Gondar ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Amhara ! ! ! ! ! ! ! ! ! Zone 1 (Awsi Rasu) ! ! ! ! ! ! ! ! ! ! ! ! ! lence, intra-community/ clan tensions, and so- ! ! ! ! ! -

Enabling Successful Migration for Youth in Addis Ababa Quantitative Survey
Enabling Successful Migration for Youth in Addis Ababa Quantitative Survey Z1 Respondent Code Sequential number according to order of the respondent (101, 102, 103, etc.) Z2 Interviewer Code Z3 Date of Interview (DD/MM/YY) / / 1 8 Z4 Location Code Based on site Z5 Start time of Interview (according to Western clock) : CONSENT: Did you complete the entire verbal script for consent, answer all questions from the respondent, and and receive a fully informed consent to participate? Investigator name: Investigator Signature: Are you willing to go ahead? Yes → continue to A1 No → stop the survey, thank the respondent, and approach another potential respondent 1 I. DEMOGRAPHIC CHARACTERISTICS A1 How old are you? Enter age A2 Sex 0 = male 1 = female 8 = don’t know 9 = refused to answer A3 Where in Ethiopia are you from? Enter code for region Enter code for zone Enter name of woreda Enter name of kebele A4 Where in Addis Ababa do you live? Enter code for subcity Enter name of district A5 Which year did you first arrive in Enter year (according to Western Addis? calendar, not Ethiopian) A6 How much time (cumulative) have Enter total number of months you spent in Addis Ababa? A7 What is your marital status? 1 = married 2 = engaged, 3 = in a partnership 4 = single 5 = widowed 6 = divorced/separated 8 = don’t know 9 = refused to answer A8 Do you have any children? 0 = no 1 = yes 8 = don’t know 9 = refused to answer A9 What is your religion? 1 = Ethiopian Orthodox 2 = Muslim 3 = Protestant 4 = Traditional/local 5 = Catholic 6 = Other 8 = Don’t know -

Ethiopia: Humanitarian Access Situation Report
ETHIOPIA Humanitarian Access Situation Report January - March 2020 This report is produced by OCHA Ethiopia in collaboration with humanitarian partners. It covers the period January to March 2020. The next report will be issued in June 2020. OVERVIEW • The operational environment to relief operations North Number of incidents by woreda Western Central remained permissive through the reporting period. Western TIGRAY Eastern 1 - 2 3 - 4 5 - 6 South Kilbet Most access impediments continue related to hos- North Rasu Gondar Eastern Wag tilities, intra-community conflicts or social unrest, Central Southern Gondar Hamra West Fantana hindering the quality of the humanitarian response, Gondar AMHARA North Wello Rasu AFAR and to COVID-19. South Awsi Gondar Rasu Metekel Hari Awi West East South Wello Gojam Rasu • Humanitarian partners are committed to support BENISHANGUL Gojam Oromia GUMUZ Siti the government response to COVID-19 and ensure North North Gabi Kemashi Horo Shewa DIRE DAWA West Shewa Rasu that critical activities are sustained. Partners are Gudru West Mao Komo Wellega Wellega Shewa Fafan Special East Addis Ababa actively implementing precautionary measures to Wellega HARARI Kelem Wellega East South West West ensure the safety of aid personnel and the popula- Buno Bedele East Hararge Hararge Ilu Aba Shewa Shewa Guraghe GAMBELA Bora Jarar tion. Nuwer Arsi Erer Agnewak Jimma Hadiya Siltie Sheka Yem Sp.Halaba Sp. OROMIA Kembata Mejenger Kefa Doolo Dawuro Tibaro Nogob SOMALI • The humanitarian community is working with gov- Bench Maji West Arsi Konta Sp. Wolayita Bale Gofa Sidama ernment counterparts to ensure that partners can Gamo Korahe Mirab Basketo Gedeo continue movements and operations throughout Omo Amaro SNNP Derashe Alle Guji Shabelle the country, bearing in mind restrictions to contain South Omo BurjiWest Guji Konso Afder the spread of COVID-19. -

Ethiopia Access Snapshot - Afar Region and Siti Zone, Somali Region As of 31 January 2020
Ethiopia Access Snapshot - Afar region and Siti zone, Somali region As of 31 January 2020 Afar region is highly prone to natural disasters Afdera The operating environment is highly compromised, with a high such as droughts and seasonal flooding. Long-- risk for humanitarian operations of becoming politicized. In ErebtiDalol Zone 2 term historical grievances coupled with Bidu March 2019, four aid workers were detained by Afar authorities TIGRAY resource-based tensions between ethnic Afar for having allegedly entered the region illegally. They were KunnebaBerahile and its neighbors i.e. Issa (Somali), and Oromo Megale conducting a humanitarian activity in Sitti zone, and decided to Teru Ittu (Amibara woreda) and Karayu (Awash Fentale woreda) in ERITREA overnight in a village of Undufo kebele. In a separate incident, in Yalo AFAR Kurri Red Sea October 2019, an attack by unidentified armed men in Afambo zone 3, and in areas adjacent to Oromia special zone and Amhara- Afdera Robe Town Aso s a Ethnic Somali IDPZone 2016/2018 4 Zone 2 (Kilbet Rasu) Elidar region, continue to cause casualties and forced displacement, Aba 'Ala woreda, Zone 1, near Djibouti, killed a number of civilians spark- Gulina Goba Town limiting partners’ movements and operations. Overall, Ethnican Oromia IDP 2016/2018 L. Afrera Ye'ch'ew ing outrage across the region and prompting peaceful demon- Awra estimated 50,000 people remain displaced, the majority of whom Erebti strations and temporarily road blockages of the Awash highway SNNP Zone 1 Bidu rely almost entirely on assistance provided by host communities. Semera TIGRAYEwa On the other hand, in 2019, the overflow of Awash River and DJIBOUTI Clashes involving Afar and Somali Issa clan continue along Megale Afele Kola flash floods displaced some 3,300 households across six Dubti boundary areas between Afar’s zone 1 and 3 and Sitti zone. -

Urgent Need for Integrated Emergency Response in 141 Outbreak and Undernutrition Affected Woredas of Ethiopia in 2020
JOINT INTER-CLUSTER ADVOCACY PAPER URGENT NEED FOR INTEGRATED EMERGENCY RESPONSE IN 141 OUTBREAK AND UNDERNUTRITION AFFECTED WOREDAS OF ETHIOPIA IN 2020 Ethiopian population is currently facing new crises while past crises are not yet resolved. The recurrent food & undernutrition crises and increased frequency of measles and cholera outbreaks in the course of 2019 are now further exacerbated by desert locust infestations and upcoming COVID-19 pandemic. The complexification of the emergency needs requires to bring together our integrated efforts and expertise to address them. Ethiopia Health Cluster, WASH Cluster and Emergency Nutrition Coordination Unit established Health, WASH and Nutrition Technical Woking Group (HWN TWG) to further boost joint response in 141 woreda and IDP camps of Ethiopia by implementing minimum multi-sectoral response package. OUR ASKS • The HWN TWG asks the EHCT to support our initiative by providing more visibility and attention to this integrated initiative and request an opportunity to provide periodic updates at EHCT meetings. • The HWN TWG asks the Government of Ethiopia, especially NDRMC, MOH, EPHI and MOWIE, to improve the joint Health, WASH and Nutrition collaboration, to foster joint system strengthening and data sharing with humanitarian actors. • The HWN TWG asks Donors, including EHF and all the Rapid Response Mechanisms, to prioritise multisectoral funding in priority woredas and consider the multi-sectoral approach as an example of good practice. • The HWN TWG asks implementing partners to include the pilot of the Health WASH and Nutrition minimum package in their future proposals and project design. Partners are encouraged to work as a consortium to jointly implement the full package. -

ETHIOPIA: Floods Update No.3 As of 18 August 2020
ETHIOPIA: Floods Update No.3 As of 18 August 2020 Overview As per the kiremt season (June – September) 2020 kiremt weather outlook weather forecast by the National According to the National Meteorological Agency kiremt (June – Meteorological Agency, all kiremt rain- receiving areas are getting normal to above September) rainy season weather outlook: normal rainfall. The heavy rainfall and • The season will have discharge of filled dams in some areas, have a timely onset and caused flooding and landslides, displacing timely cessation. people in several parts of the country. • High likelihood of In western Ethiopia, heavy rainfalls have led to wetter climate flooding in the lowlands of Gambella region and (especially in July landslide in East Wollega zone, Oromia region. and August) in the south western, In eastern Ethiopia, flooding occurred in western and central Shabelle and Siti zones, Somali region in early August. At least 26 woredas across the region parts of the country. were already affected by flooding in April 2020. • Dominantly normal rainfall in eastern In northern Ethiopia, heavy rains in the Ethiopia. highlands of Amhara and Tigray regions, the • Heavy rainfall will likely cause flooding and landslides in flood- backflow of the Tendaho dam and the overflow prone areas. of Awash River continue to pose heightened • Timely onset and good performance of the rains will overall benefit risk of flooding in Afar region during the remainder of the kiremt season. At least 13 agricultural activities (planting of short maturing crops), pasture woredas were already affected by flooding in regeneration and water replenishment. Afar, displacing 40,731 people in July and • According to the Ministry of Water and Energy, the level of dam August.