Figure 1. Structural Diversification of Pyrethroid Insecticides. Early Synthetic Pyrethroids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Polystyrene Microplastics Do Not Affect Juvenile Brown Trout (Salmo Trutta F
Schmieg et al. Environ Sci Eur (2020) 32:49 https://doi.org/10.1186/s12302-020-00327-4 RESEARCH Open Access Polystyrene microplastics do not afect juvenile brown trout (Salmo trutta f. fario) or modulate efects of the pesticide methiocarb Hannah Schmieg1*, Sven Huppertsberg2, Thomas P. Knepper2, Stefanie Krais1, Katharina Reitter1, Felizitas Rezbach1, Aki S. Ruhl3,4, Heinz‑R. Köhler1 and Rita Triebskorn1,5 Abstract Background: There has been a rising interest within the scientifc community and the public about the environmen‑ tal risk related to the abundance of microplastics in aquatic environments. Up to now, however, scientifc knowledge in this context has been scarce and insufcient for a reliable risk assessment. To remedy this scarcity of data, we inves‑ tigated possible adverse efects of polystyrene particles (10 4 particles/L) and the pesticide methiocarb (1 mg/L) in juvenile brown trout (Salmo trutta f. fario) both by themselves as well as in combination after a 96 h laboratory expo‑ sure. PS beads (density 1.05 g/mL) were cryogenically milled and fractionated resulting in irregular‑shaped particles (< 50 µm). Besides body weight of the animals, biomarkers for proteotoxicity (stress protein family Hsp70), oxidative stress (superoxide dismutase, lipid peroxidation), and neurotoxicity (acetylcholinesterase, carboxylesterases) were analyzed. As an indicator of overall health, histopathological efects were studied in liver and gills of exposed fsh. Results: Polystyrene particles by themselves did not infuence any of the investigated biomarkers. In contrast, the exposure to methiocarb led to a signifcant reduction of the activity of acetylcholinesterase and the two carboxy‑ lesterases. Moreover, the tissue integrity of liver and gills was impaired by the pesticide. -

Bayesian Nonparametric Model for Clustering Individual Co-Exposure to Pesticides Found in the French Diet
Bayesian nonparametric model for clustering individual co-exposure to pesticides found in the French diet. Amélie Crépet, Jessica Tressou To cite this version: Amélie Crépet, Jessica Tressou. Bayesian nonparametric model for clustering individual co-exposure to pesticides found in the French diet.. 2009. hal-00438796v2 HAL Id: hal-00438796 https://hal.archives-ouvertes.fr/hal-00438796v2 Preprint submitted on 12 Jan 2011 (v2), last revised 4 Feb 2011 (v3) HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Bayesian nonparametric model for clustering individual co-exposure to pesticides found in the French diet. Am´elieCr´epet a & Jessica Tressoub January 12, 2011 aANSES, French Agency for Food, Environmental and Occupational Health Safety, 27-31 Av. G´en´eralLeclerc, 94701 Maisons-Alfort, France bINRA-Met@risk, Food Risk Analysis Methodologies, National Institute for Agronomic Re- search, 16 rue Claude Bernard, 75231 Paris, France Keywords Dirichlet process; Bayesian nonparametric modeling; multivariate Normal mixtures; clustering; multivariate exposure; food risk analysis. Abstract This work introduces a specific application of Bayesian nonparametric statistics to the food risk analysis framework. The goal was to determine the cocktails of pesticide residues to which the French population is simultaneously exposed through its current diet in order to study their possible combined effects on health through toxicological experiments. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

R Eaction Pathways and Mechanisms of Photodegradation of Pesticides H.D
Journal of Photochemistry and Photobiology B: Biology 67 (2002) 71±108 www.elsevier.com/locate/jphotobiol Invited Review R eaction pathways and mechanisms of photodegradation of pesticides H.D. Burrowsab,bc , M. Canle L* , J.A. Santaballa , S. Steenken aDepartamento de Quõmica , Universidade de Coimbra, 3004-535 Coimbra, Portugal bDepartamento de Quõmica Fõsica e EnxenerõaÄÄ Â Quõmica I, Universidade da Coruna, Rua  Alejandro de la Sota 1, E-15008 A Coruna Ä, Galicia, Spain cMax-Planck Institut f urÈÈ Strahlenchemie, D-45413 Mulheim an der Ruhr, Germany Received 26 March 2001; accepted 25 February 2002 Abstract The photodegradation of pesticides is reviewed, with particular reference to the studies that describe the mechanisms of the processes involved, the nature of reactive intermediates and ®nal products. Potential use of photochemical processes in advanced oxidation methods for water treatment is also discussed. Processes considered include direct photolysis leading to homolysis or heterolysis of the pesticide, photosensitized photodegradation by singlet oxygen and a variety of metal complexes, photolysis in heterogeneous media and degradation by reaction with intermediates generated by photolytic or radiolytic means. 2002 Elsevier Science B.V. All rights reserved. Keywords: Pesticides; Photodegradation; Photoionization; Photocatalysis; Photosensitization; Photolysis; Hydroxyl radicals 1 . Introduction worsen with the appearance of new and more sophisticated substances. Due to the world-wide general application of intensive The critical nature of this environmental problem has agricultural methods during the last few decades and to the prompted the development of faster and more accurate large-scale development of the agrochemical industry, the methods for the characterisation and quanti®cation of the variety and quantities of agrochemicals present in con- pesticides dispersed in the environment. -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -

The Insecticides Act, 1968 (Act No.46 of 1968)
The Insecticides Act, 1968 (Act No.46 of 1968) An Act to regulate the import, manufactures, sale, transport, distribution and use of insecticides with a view to prevent risk to human beings or animals and for matters connected therewith. [2 nd September 1968] Be it enacted by Parliament in the Nineteenth Year of the Republic of India as follows: 1. Short title, extent and commencement. * a. This Act may be called the Insecticides Act, 1968. b. It extends to the whole of India. c. It shall come into force on such date as the Central Government may, by notification in the official Gazette, appoint and different dates may be appointed for different States and for different provisions of Act. 2. Application of other laws not barred * The provisions of this Act shall be in addition to, and not in derogation of, any other law for the time being in force. 3. Definitions- In this Act, unless the context otherwise requires- a. "animals" means animals useful to human beings and includes fish and fowl, and such kinds of wild life as the Central Government may, by notification in the official Gazette, specify, being kinds which in its opinion, it is desirable to protect or preserve; b. "Board" means the Central Insecticides Board constituted under Sec.4; c. "Central Insecticides Laboratory" means the Central Insecticides Laboratory established, or as the case may be, the institution specified under Sec.16; d. "Import" means bringing into any place within the territories to which this Act extends from a place outside those territories; e. "Insecticide" means- i. -
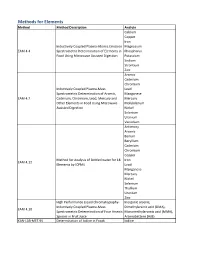
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and -

The 2018 European Union Report on Pesticide Residues in Food
SCIENTIFIC REPORT ADOPTED: 24 February 2020 doi: 10.2903/j.efsa.2020.6057 The 2018 European Union report on pesticide residues in food European Food Safety Authority (EFSA), Paula Medina-Pastor and Giuseppe Triacchini Abstract Under EU legislation (Article 32, Regulation (EC) No 396/2005), EFSA provides an annual report which analyses pesticide residue levels in foods on the European market. The analysis is based on data from the official national control activities carried out by EU Member States, Iceland and Norway and includes a subset of data from the EU-coordinated control programme which uses a randomised sampling strategy. For 2018, 95.5% of the overall 91,015 samples analysed fell below the maximum residue level (MRL), 4.5% exceeded this level, of which 2.7% were non-compliant, i.e. samples exceeding the MRL after taking into account the measurement uncertainty. For the subset of 11,679 samples analysed as part of the EU-coordinated control programme, 1.4% exceeded the MRL and 0.9% were non-compliant. Table grapes and sweet peppers/bell peppers were among the food products that most frequently exceeded the MRLs. To assess acute and chronic risk to consumer health, dietary exposure to pesticide residues was estimated and compared with health-based guidance values. The findings suggest that the assessed levels for the food commodities analysed are unlikely to pose concern for consumer health. However, a number of recommendations are proposed to increase the efficiency of European control systems (e.g. optimising traceability), thereby continuing to ensure a high level of consumer protection. -

Usive Use Period for Spinetoram
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON D.C., 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION OCT 1 8 2016 MEMORANDUM SUBJECT: Review ofa Request for an Extension ofthe Exclusive Use Period for Spinetoram FROM: Caleb Hawkins, Biologist /J. I. ~~ Biological Analysis Branch ~' Biological and Economic Analysis Division ( 503P) THRU: Monisha Kaul, Chief Biological Analysis Branch Biological and Economic Analysis Division (7503P) TO: Carlyn Petrella, Biologist Invertebrate Branch II (IVB2) Registration Division (7505P) BEAD Product Review Panel date: August 24, 2016 SUMMARY When a new pesticide active ingredient is registered, the original data submitter has a 10-year period following the date ofregistration during which they retain exclusive use ofthe data. FIFRA allows for an extension of that exclusive-use period for up to three years if minor uses that meet certain criteria are registered. A one-year extension is allowed for every three qualifying minor uses. Dow AgroSciences submitted a petition to EPA requesting that, under FIFRA Section 3(c)(l)(F)(ii), the exclusive use period for data supporting the active ingredient spinetoram be extended for three years. Because three minor uses must meet the criteria for each one-year extension, nine registered minor uses are required to meet the criteria for a three-year extension. Supporting information was submitted for nine crops that the registrant identified as minor uses meeting the criteria established for extension of exclusive use. BEAD evaluated the available data on the number ofacres ofproduction for each of the crops listed by the registrant to assess whether the crops meet the definition ofminor use per FIFRA Section 2(11)(1) and then applied the benefits-related criteria for extension ofexclusive use expressed in FIFRA Section 3(c)(l)(F)(ii) to the crops proposed by the registrant. -

PAN International List of Highly Hazardous Pesticides
PAN International List of Highly Hazardous Pesticides (PAN List of HHPs) December 2016 • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • Pesticide Action Network International Impressum © PAN International c/o PAN Germany, Nernstweg 32, 22765 Hamburg, Germany December, 2016 This 'PAN International List of Highly Hazardous Pesticides' was initially drafted by PAN Germany for PAN International. The 1st version was adopted by PAN International 2008 and published January 2009. Since then the list has been updated several times as classifications changed for numerous individual pesticides. In 2013/2014 the PAN International Working Group on “HHP criteria” revised the criteria used in this list to identify highly hazardous pesticides. This December 2016 version of the list is based on these hazard criteria adopted by PAN International in June 2014. • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • Contents Background and introduction ................................................................................................. 4 About this List ........................................................................................................................ 8 What is new in this List ........................................................................................................ 10 Work in progress ................................................................................................................ -
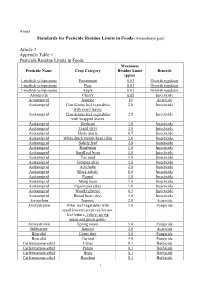
Standards for Pesticide Residue Limits in Foods (Amendment Part)
Annex Standards for Pesticide Residue Limits in Foods (Amendment part) Article 3 Appendix Table 1 Pesticide Residue Limits in Foods Maximum Pesticide Name Crop Category Residue Limit Remark (ppm) 1-methylcyclopropene Persimmon 0.01 Growth regulator 1-methylcyclopropene Pear 0.01 Growth regulator 1-methylcyclopropene Apple 0.01 Growth regulator Abamectin Cherry 0.09 Insecticide Acequinocyl Jasmine 10 Acaricide Acetamiprid Cruciferous leaf vegetables 2.0 Insecticide with small leaves Acetamiprid Cruciferous leaf vegetables 2.0 Insecticide with wrapped leaves Acetamiprid Soybean 1.0 Insecticide Acetamiprid Lentil (dry) 1.0 Insecticide Acetamiprid Hairy uraria 0.5 Insecticide Acetamiprid White dutch runner bean (dry) 1.0 Insecticide Acetamiprid Salsify leaf 2.0 Insecticide Acetamiprid Rambutan 1.0 Insecticide Acetamiprid Small red bean 1.0 Insecticide Acetamiprid Tea seed 1.0 Insecticide Acetamiprid Cowpea (dry) 1.0 Insecticide Acetamiprid Artichoke 2.0 Insecticide Acetamiprid Black salsify 0.5 Insecticide Acetamiprid Peanut 1.0 Insecticide Acetamiprid Mung bean 1.0 Insecticide Acetamiprid Pigeon pea (dry) 1.0 Insecticide Acetamiprid Woolly glycine 0.5 Insecticide Acetamiprid Broad bean (dry) 1.0 Insecticide Acrinathrin Jasmine 2.0 Acaricide Azoxystrobin Other leaf vegetables with 1.0 Fungicide small leaves(except cos lettuce, leaf lettuce, celery, spring onion and green garlic) Azoxystrobin Spring onion 5.0 Fungicide Bifenazate Jasmine 2.0 Acaricide Boscalid Citrus fruit 5.0 Fungicide Boscalid Currant 4.0 Fungicide Carfentrazone-ethyl Citrus