Kernowite, Cu2fe(Aso4)(OH)4⋅4H2O, the Fe -Analogue of Liroconite from Cornwall, UK
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Liroconite Cu2al(Aso4)(OH)4 • 4H2O C 2001-2005 Mineral Data Publishing, Version 1
Liroconite Cu2Al(AsO4)(OH)4 • 4H2O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic. Point Group: 2/m. Typically as crystals with a flattened octahedral or lenticular aspect, dominated by {110} and {011} and striated parallel to their intersections, also {001}, {010}, {100}, to 3.6 cm, alone and in sub-parallel groups. May be granular, massive. Physical Properties: Cleavage: On {110}, {011}, indistinct. Fracture: Uneven to conchoidal. Hardness = 2–2.5 D(meas.) = 2.94–3.01 D(calc.) = [3.03] Optical Properties: Transparent to translucent. Color: Sky-blue, bluish green, verdigris-green, emerald-green; pale blue to pale bluish green in transmitted light. Streak: Pale blue to pale green. Luster: Vitreous to resinous. Optical Class: Biaxial (–). Orientation: Y = b; Z ∧ a =25◦. Dispersion: r< v,moderate. α = 1.612(3) β = 1.652(3) γ = 1.675(3) 2V(meas.) = n.d. 2V(calc.) = 72(5)◦ Cell Data: Space Group: I2/a. a = 12.664(2) b = 7.563(2) c = 9.914(3) β =91.32(2)◦ Z=4 X-ray Powder Pattern: Cornwall, England. 6.46 (10), 3.01 (10), 5.95 (9), 2.69 (6), 3.92 (5), 2.79 (5), 2.21 (5) Chemistry: (1) (2) P2O5 3.73 As2O5 23.05 26.54 Al2O3 10.85 11.77 Fe2O3 0.98 CuO 36.38 36.73 H2O 25.01 24.96 Total 100.00 100.00 • (1) Cornwall, England. (2) Cu2Al(AsO4)(OH)4 4H2O. Occurrence: A rare secondary mineral in the oxidized zone of some copper deposits. Association: Olivenite, chalcophyllite, clinoclase, cornwallite, strashimirite, malachite, cuprite, “limonite”. -

RICHARD W. BARSTOW 26, Tregeseal, St. Just, Near Penzance. Cornwall. England. ORDERING INFORMATION Mail Orders Are Prollipt~Y Fi
RICHARD W. BARSTOW 26, Tregeseal, st. Just, Near Penzance. Cornwall. England. ORDERING INFORMATION Mail orders are prollipt~y filled and despatched on a 7-day examination basis, subject to approval. Immediate refund guaranteed on return of specimens. Please quote the name and the number of the specimen(s) required, and enclose P.O./Cheque with order. No charge is made for postage and packing, except for over seas customers and postage over 50p. We reserve the ~ight to make slight substitutions, if necessary, unless advised to the contrary. Special requests and 'wants lists' are welcome. We hope that we may be of some service to you, and assure you of our best attention at all times. JULY 1973 1. ADAMITE. Ojuela Mine, Mapimi, Mexico. Choice well-formed yellowish green doubly terminated crystals richly encrusting cellular Limonite matrix, with minor Calcite lT in association. 3x2t • £5. 2. ANDRADITE GARNET variety DEMANTOID. Val Malenco,Sondrio, Italy. Well formed sharp apple green crystals richly scattered over Serpentinite. 2txlt". £6. 3. APATITE. Colcerrow Quarry, Luxulyan, Cornwall. Very fine highly modified clear sea green crystals to a tIT in size intergrown and scattered over Pegmatite matrix, with minor Gilbertite mica in association. Six specimens are lT lT being offered varying in size from lxl - lxlt , all show excellent Apatite crYstals, and are taken from an old collection. Prices from £1.50 - £2.50 dependent on quality and size. ~. NATIVE ARSENIC. Grube Sampson, 8'1>. Andreasberg, Harz Mts., Germany. Unusual silvery grey It" botryoidal mass. £~. ANGLESITE. Wheal Penrose, Porthleven, Cornwall. Fine clear glassy crystals to ~ mm. -
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Cornwall Council Altarnun Parish Council
CORNWALL COUNCIL THURSDAY, 4 MAY 2017 The following is a statement as to the persons nominated for election as Councillor for the ALTARNUN PARISH COUNCIL STATEMENT AS TO PERSONS NOMINATED The following persons have been nominated: Decision of the Surname Other Names Home Address Description (if any) Returning Officer Baker-Pannell Lisa Olwen Sun Briar Treween Altarnun Launceston PL15 7RD Bloomfield Chris Ipc Altarnun Launceston Cornwall PL15 7SA Branch Debra Ann 3 Penpont View Fivelanes Launceston Cornwall PL15 7RY Dowler Craig Nicholas Rivendale Altarnun Launceston PL15 7SA Hoskin Tom The Bungalow Trewint Marsh Launceston Cornwall PL15 7TF Jasper Ronald Neil Kernyk Park Car Mechanic Tredaule Altarnun Launceston Cornwall PL15 7RW KATE KENNALLY Dated: Wednesday, 05 April, 2017 RETURNING OFFICER Printed and Published by the RETURNING OFFICER, CORNWALL COUNCIL, COUNCIL OFFICES, 39 PENWINNICK ROAD, ST AUSTELL, PL25 5DR CORNWALL COUNCIL THURSDAY, 4 MAY 2017 The following is a statement as to the persons nominated for election as Councillor for the ALTARNUN PARISH COUNCIL STATEMENT AS TO PERSONS NOMINATED The following persons have been nominated: Decision of the Surname Other Names Home Address Description (if any) Returning Officer Kendall Jason John Harrowbridge Hill Farm Commonmoor Liskeard PL14 6SD May Rosalyn 39 Penpont View Labour Party Five Lanes Altarnun Launceston Cornwall PL15 7RY McCallum Marion St Nonna's View St Nonna's Close Altarnun PL15 7RT Richards Catherine Mary Penpont House Altarnun Launceston Cornwall PL15 7SJ Smith Wes Laskeys Caravan Farmer Trewint Launceston Cornwall PL15 7TG The persons opposite whose names no entry is made in the last column have been and stand validly nominated. -

Chapter 4 Minerals (Quiz Free)
Minerals Chapter 4 Section 4.1 What is a mineral? Define a mineral. Describe how minerals form. Identify the most common elements in Earth’s crust. Potash Liroconite Sphalerite Gold Earth’s Crust There are at least 3,000 known minerals in Earth’s crust. Define a mineral. A Mineral – (characteristics)is •naturally occurring •inorganic solid •has a crystal structure •definite chemical composition. Potash Liroconite Gold Sphalerite (Native Element) Solid Minerals always exist in a solid form. Salt Diamond Composition Although a few minerals are composed of single elements, most are made from compounds. Gold Sphalerite Liroconite Potash Composition (continued) Solids with a specific chemical composition Quartz’s chemical ratio (recipe) is always: SiO2 Composition may vary slightly Quartz within a well-defined range. The recipe is still the same. Olivine (Mg,Fe) SiO 100% 2 4 100% Mg Fe Olivine Forsterite Fayalite Magma Magma - Molten material found beneath Earth’s crust Minerals formation Minerals can form when differences in density force magma upward into cooler layers of Earth’s interior. Minerals from solution Minerals form from cooled magma and from elements in solutions. Minerals from solution (Continued) Mineral crystals may begin to precipitate out of a solution that has become saturated. Most abundant elements The most abundant elements in Earth’s crust are oxygen and silicon . Most common minerals The most common minerals, feldspar and quartz, are silicates. (SiO ) tetrahedron XAl(1-2) Si(3-2) O8 4 X may = Sodium, potassium, calcium Silicates Silicate - Mineral that contains silicon and oxygen (SiO4) tetrahedron 4.1 – What is a Mineral? Quiz 1.Although a few minerals are composed of single elements most are made from ____________. -

Election of Parish Councillors for Altarnun Parish Council on Thursday 6 May 2021
RETURN OF RESULT OF UNCONTESTED ELECTION Cornwall Council Election of Parish Councillors for Altarnun Parish Council on Thursday 6 May 2021 I, Kate Kennally, being the Returning Officer for the Parish of ALTARNUN PARISH COUNCIL at an Election of Parish Councillors for the said Parish report that the latest time for delivery of notices of withdrawal of candidature, namely Thursday 8 April 2021, having passed, the persons whose names appear in the accompanying list stood validly nominated and were duly elected without a contest. Name of Candidate Home Address Description (if any) BLOOMFIELD (address in Cornwall) Chris BRANCH 3 Penpont View, Five Lanes, Debra Ann Altarnun, Launceston, Cornwall, PL15 7RY COLES 17 St Nonnas Close, Altarnun, Lauren Launceston, PL15 7RU DOWLER (address in Cornwall) Craig Nicholas GREEN The Dovecote, Tredoggett Farm, Carl Stuart Altarnun, Launceston, Cornwall, PL15 7SA HOSKIN The Bungalow, Trewint Marsh, Tom Launceston, Cornwall, PL15 7TF KENDALL (address in Cornwall) Jason John MARSH 1 Todda Close, Bolventor, PL15 Health And Social Care Managing Leah Michelle 7FP Director SMITH (address in Cornwall) Polly Jane SMITH (address in Cornwall) Wesley Arthur Dated Tuesday 13 April 2021 Kate Kennally Returning Officer Printed and published by the Returning Officer, 3rd Floor, South Wing, County Hall, Treyew Road, Truro, TR1 3AY RETURN OF RESULT OF UNCONTESTED ELECTION Cornwall Council Election of Parish Councillors for Antony Parish Council on Thursday 6 May 2021 I, Kate Kennally, being the Returning Officer for the Parish of ANTONY PARISH COUNCIL at an Election of Parish Councillors for the said Parish report that the latest time for delivery of notices of withdrawal of candidature, namely Thursday 8 April 2021, having passed, the persons whose names appear in the accompanying list stood validly nominated and were duly elected without a contest. -

Journal of the Russell Society, Vol 4 No 2
JOURNAL OF THE RUSSELL SOCIETY The journal of British Isles topographical mineralogy EDITOR: George Ryba.:k. 42 Bell Road. Sitlingbourn.:. Kent ME 10 4EB. L.K. JOURNAL MANAGER: Rex Cook. '13 Halifax Road . Nelson, Lancashire BB9 OEQ , U.K. EDITORrAL BOARD: F.B. Atkins. Oxford, U. K. R.J. King, Tewkesbury. U.K. R.E. Bevins. Cardiff, U. K. A. Livingstone, Edinburgh, U.K. R.S.W. Brai thwaite. Manchester. U.K. I.R. Plimer, Parkvill.:. Australia T.F. Bridges. Ovington. U.K. R.E. Starkey, Brom,grove, U.K S.c. Chamberlain. Syracuse. U. S.A. R.F. Symes. London, U.K. N.J. Forley. Keyworth. U.K. P.A. Williams. Kingswood. Australia R.A. Howie. Matlock. U.K. B. Young. Newcastle, U.K. Aims and Scope: The lournal publishes articles and reviews by both amateur and profe,sional mineralogists dealing with all a,pecI, of mineralogy. Contributions concerning the topographical mineralogy of the British Isles arc particularly welcome. Not~s for contributors can be found at the back of the Journal. Subscription rates: The Journal is free to members of the Russell Society. Subsc ription rates for two issues tiS. Enquiries should be made to the Journal Manager at the above address. Back copies of the Journal may also be ordered through the Journal Ma nager. Advertising: Details of advertising rates may be obtained from the Journal Manager. Published by The Russell Society. Registered charity No. 803308. Copyright The Russell Society 1993 . ISSN 0263 7839 FRONT COVER: Strontianite, Strontian mines, Highland Region, Scotland. 100 mm x 55 mm. -
Surfacing & Patching Works
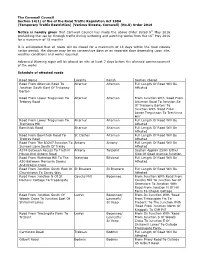
The Cornwall Council Section 14(1) of the of the Road Traffic Regulation Act 1984 (Temporary Traffic Restriction) (Various Streets, Cornwall) (No.8) Order 2016 Notice is hereby given that Cornwall Council has made the above Order dated 9th May 2016 prohibiting the use by through traffic during surfacing and patching works from the 16 th May 2016 for a maximum of 18 months It is anticipated that all roads will be closed for a maximum of 14 days within the road closure notice period; the closure may be on consecutive days or on separate days depending upon site, weather conditions and works required. Advanced Warning signs will be placed on site at least 7 days before the planned commencement of the works Schedule of affected roads Road Name Locality Parish Section Closed Road From Altarnun Road To Altarnun Altarnun Full Length Of Road Will Be Junction South East Of Trelawny Affected Barton Road From Lower Tregunnon To Altarnun Altarnun From Junction With 'Road From Trebray Road Altarnun Road To Junction Se Of Trelawny Barton' To Junction With 'Road From Lower Tregunnon To Trethinna Hill' Road From Lower Tregunnon To Altarnun Altarnun Full Length Of Road Will Be Trethinna Hill Affected Bowithick Road Altarnun Altarnun Full Length Of Road Will Be Affected Road From Bowithick Road To St Clether Altarnun Full Length Of Road Will Be Trebray Road Affected Road From The B3247 Junction To Antony Antony Full Length Of Road Will Be Sunwell Lane South Of Trelay Affected A374 Between Access To Trethill Antony Torpoint Section Approx 250m Either House -

NO. 7. RESTORMELITE. I Have Obtained on Various Ocasions, from the Restormel Iron Mine, a Mineral Which Might Easilybe Mistaken for One Or Other View Article Online
View Article Online / Journal Homepage / Table of Contents for this issue CHURCH’S CHEMICAL RESEARCHES, ETC. 165 Published on 01 January 1870. Downloaded by University of Lancaster 30/10/2014 18:23:36. XXK- C7iemicaI Researches on hTew or Rare Cornish Minerals. By A. H. CHURCH,M.A., Oxon., Professor of Chemistry, Royal Agricultural College, Cirencester. NO. 7. RESTORMELITE. I have obtained on various ocasions, from the Restormel Iron Mine, a mineral which might easilybe mistaken for one or other View Article Online 166 CHURCH’S CHEMICAL RESEARCHES of the Chinese stones, which are grouped under the vague term agalmatolite. But on comparing the physical and chemical characters of these oriental pinites and pyrophyllites with my Cornish specimeiis, I found such marked differences as to induce me to make a more extended study of the subject. The mate- rial being amorphous, and evidently incompletely altered, was far from promising, but the hope of throwing some light upon the chemical history and formation of similar products, induced me to make several analyses of my specimens. The constancy of their characters and composition was distinctly proved by a physical examination, and by the experimental cheniical results given below. I do not, however, think that I should be in the least degree warranted in assigning specific rank to the Restor- me1 mineral, and though I propose for it the definite name of restorinelite, its proper place appears to me that of a new variety of kaolinite. If we look at the various hydrated aluminium silicates, which have been separately recognized, we shall find that restormelite is near halloysite, but differs from that species by containing 7 per cent. -

County Geology Site Criteria for Cornwall and the Isles of Scilly
Heading County Geology Site Criteria for Cornwall and the Isles of Scilly Environmental Records Centre for Cornwall and the Isles of Scilly We are very grateful to all those who provided input and comments during the production of this document. Particular thanks go to Peter Ealey and the other members of the Cornwall RIGS Group who willingly shared their knowledge and expertise and whose contribution has been invaluable. ERCCIS and Cornwall Wildlife Trust (2010) County Geology Site Criteria for Cornwall and the Isles of Scilly Written by Susan Hocking, with contributions from the Cornwall RIGS Group Edited by Victoria Whitehouse, Cheryl Marriott and Fay Robinson Designed by Sheila McCann-Downes Drawings by Sarah McCartney Copies can be obtained from: ERCCIS c/o Cornwall Wildlife Trust Five Acres Allet Truro TR4 9DJ [email protected] COUNTY GEOLOGY SITE CRITERIA FOR CORNWALL AND THE ISLES OF SCILLY CONTENTS 1 Introduction 1 2 Context and Background 2 2.1 The Geological Heritage of Cornwall and the Isles of Scilly 2 2.2 Sites of National Importance 3 2.3 Sites of Local Importance 3 2.4 Background to County Geology Sites/RIGS in Cornwall 3 3 County Geology Site Selection 4 3.1 The Role of the JNCC (1977) Guidelines 4 3.2 Nature Conservancy Council (NCC) (1990) Guidelines 4 3.3 Framework for Establishing the County Geology Site/RIGS System 5 3.4 Guiding Principles for County Geology Site/RIGS Selection 5 3.5 Procedures for County Geology Site/RIGS Selection 7 3.6 Site Boundaries 8 4 Criteria for County Geology Site/RIGS Selection in Cornwall & The Isles of Scilly 9 BIBLIOGRAPHY 12 APPENDICES Appendix 1 – List of County Geology Sites/RIGS in Cornwall & The Isles of Scilly Appendix 2 – The Geological Conservation Review (GCR) Background to Site Selection Appendix 3 – Example of a County Geology Site/RIGS Summary Sheet Appendix 4 – Protocol for amending County Geology Site/RIGS boundaries (DRAFT) 1. -

A Specific Gravity Index for Minerats
A SPECIFICGRAVITY INDEX FOR MINERATS c. A. MURSKyI ern R. M. THOMPSON, Un'fuersityof Bri.ti,sh Col,umb,in,Voncouver, Canad,a This work was undertaken in order to provide a practical, and as far as possible,a complete list of specific gravities of minerals. An accurate speciflc cravity determination can usually be made quickly and this information when combined with other physical properties commonly leads to rapid mineral identification. Early complete but now outdated specific gravity lists are those of Miers given in his mineralogy textbook (1902),and Spencer(M,i,n. Mag.,2!, pp. 382-865,I}ZZ). A more recent list by Hurlbut (Dana's Manuatr of M,i,neral,ogy,LgE2) is incomplete and others are limited to rock forming minerals,Trdger (Tabel,l,enntr-optischen Best'i,mmungd,er geste,i,nsb.ildend,en M,ineral,e, 1952) and Morey (Encycto- ped,iaof Cherni,cal,Technol,ogy, Vol. 12, 19b4). In his mineral identification tables, smith (rd,entifi,cati,onand. qual,itatioe cherai,cal,anal,ys'i,s of mineral,s,second edition, New york, 19bB) groups minerals on the basis of specificgravity but in each of the twelve groups the minerals are listed in order of decreasinghardness. The present work should not be regarded as an index of all known minerals as the specificgravities of many minerals are unknown or known only approximately and are omitted from the current list. The list, in order of increasing specific gravity, includes all minerals without regard to other physical properties or to chemical composition. The designation I or II after the name indicates that the mineral falls in the classesof minerals describedin Dana Systemof M'ineralogyEdition 7, volume I (Native elements, sulphides, oxides, etc.) or II (Halides, carbonates, etc.) (L944 and 1951). -

C:\Documents and Settings\Alan Smithee\My Documents\MOTM
@oqhk1//5Lhmdq`knesgdLnmsg9B`u`mrhsd “A trove of royal-blue cavansite specimens that astonished the mineral community at the Tucson Show in 1989 was collected from a quarry in the vicinity of Wagholi village. Thousands of fine examples, the best ever seen for the species, were found there. Additional occurrences in quarries nearby have now been discovered.” –Rustam Z. Kothavala, Mineralogical Record, November-December 1991. OGXRHB@K OQNODQSHDR Chemistry: Ca(VO)Si4O10A4H2O Hydrous Calcium Vanadium Silicate (Calcium Vanadium Silicate Hydrate), also Hydrous Calcium Vanadyl Silicate Class: Silicates Subclass: Phyllosilicates Group: Cavansite (Vanadium-containing Phyllosilicates) Crystal System: Orthorhombic Crystal Habits: Usually as isolated spherical aggregates, radiating spheres, tufts, or rosettes of crystals; less commonly as linear clusters of somewhat more distinct individual crystals; terminations pointed but not sharp; twinning rare. Almost always occurs as inclusions in, or growths on, zeolite minerals. Small crystals rarely exceed one-half inch in length. Color: Greenish blue to deep, vivid blue and “electric” blue Luster: Vitreous Transparency: Transparent to translucent Streak: Bluish-white Cleavage: Perfect in one direction Fracture: Conchoidal, brittle Hardness: 3.0-4.0, varies directionally Specific Gravity: 2.21-2.31 Luminescence: None Refractive Index: 1.54-1.55 Distinctive Features and Tests: Best field indications are vivid blue color, secondary occurrence in vesicles or fractures of basalt or andesite, and association with zeolite minerals. Cavansite is sometimes confused with the dimorphic mineral pentagonite, as explained in Composition. Dana Classification Number: 74.3.7.1 M @L D Correctly pronounced “CAV-an-site,” the mnemonic (“assisting memory”) name is derived from an acronym consisting of the first letters of three of its elemental components: CAlcium, VANadium, and SIlicon.