(Nephus) Voeltzkowi Weise (Coleoptera: Coccinellidae), with Comments on the Origin of a Neartic Population and Its Possible Asexual Status
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COLEOPTERA COCCINELLIDAE) INTRODUCTIONS and ESTABLISHMENTS in HAWAII: 1885 to 2015
AN ANNOTATED CHECKLIST OF THE COCCINELLID (COLEOPTERA COCCINELLIDAE) INTRODUCTIONS AND ESTABLISHMENTS IN HAWAII: 1885 to 2015 JOHN R. LEEPER PO Box 13086 Las Cruces, NM USA, 88013 [email protected] [1] Abstract. Blackburn & Sharp (1885: 146 & 147) described the first coccinellids found in Hawaii. The first documented introduction and successful establishment was of Rodolia cardinalis from Australia in 1890 (Swezey, 1923b: 300). This paper documents 167 coccinellid species as having been introduced to the Hawaiian Islands with forty-six (46) species considered established based on unpublished Hawaii State Department of Agriculture records and literature published in Hawaii. The paper also provides nomenclatural and taxonomic changes that have occurred in the Hawaiian records through time. INTRODUCTION The Coccinellidae comprise a large family in the Coleoptera with about 490 genera and 4200 species (Sasaji, 1971). The majority of coccinellid species introduced into Hawaii are predacious on insects and/or mites. Exceptions to this are two mycophagous coccinellids, Calvia decimguttata (Linnaeus) and Psyllobora vigintimaculata (Say). Of these, only P. vigintimaculata (Say) appears to be established, see discussion associated with that species’ listing. The members of the phytophagous subfamily Epilachninae are pests themselves and, to date, are not known to be established in Hawaii. None of the Coccinellidae in Hawaii are thought to be either endemic or indigenous. All have been either accidentally or purposely introduced. Three species, Scymnus discendens (= Diomus debilis LeConte), Scymnus ocellatus (=Scymnobius galapagoensis (Waterhouse)) and Scymnus vividus (= Scymnus (Pullus) loewii Mulsant) were described by Sharp (Blackburn & Sharp, 1885: 146 & 147) from specimens collected in the islands. There are, however, no records of introduction for these species prior to Sharp’s descriptions. -

Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas
Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas Neal L. Evenhuis, Lucius G. Eldredge, Keith T. Arakaki, Darcy Oishi, Janis N. Garcia & William P. Haines Pacific Biological Survey, Bishop Museum, Honolulu, Hawaii 96817 Final Report November 2010 Prepared for: U.S. Fish and Wildlife Service, Pacific Islands Fish & Wildlife Office Honolulu, Hawaii Evenhuis et al. — Pagan Island Arthropod Survey 2 BISHOP MUSEUM The State Museum of Natural and Cultural History 1525 Bernice Street Honolulu, Hawai’i 96817–2704, USA Copyright© 2010 Bishop Museum All Rights Reserved Printed in the United States of America Contribution No. 2010-015 to the Pacific Biological Survey Evenhuis et al. — Pagan Island Arthropod Survey 3 TABLE OF CONTENTS Executive Summary ......................................................................................................... 5 Background ..................................................................................................................... 7 General History .............................................................................................................. 10 Previous Expeditions to Pagan Surveying Terrestrial Arthropods ................................ 12 Current Survey and List of Collecting Sites .................................................................. 18 Sampling Methods ......................................................................................................... 25 Survey Results .............................................................................................................. -

Coleoptera: Coccinellidae) from the West Bank (Central Palestine)
Zootaxa 4664 (1): 001–046 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4664.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:839DFCCC-A83F-408E-99E3-7A51EFDF18D3 Systematic list, geographic distribution and ecological significance of lady beetles (Coleoptera: Coccinellidae) from the West Bank (Central Palestine) MOHAMMAD H. NAJAJRAH1,2*, KHALID M. SWAILEH1 & MAZIN B. QUMSIYEH 2 1Birziet University, Faculty of Science, Department of Biology and Biochemistry, Master’s Program in Environmental Biology, P. O. Box 14, Birzeit, West Bank, Palestine. Email: [email protected], [email protected] 2Bethlehem University, Palestine Museum of Natural History, Rue des Freres # 9, Bethlehem, West Bank, Palestine. E-mail: [email protected], [email protected] *Corresponding author. E-mail: [email protected] Abstract We surveyed and identified species of lady beetles from the West Bank to document their geographic distribution and understand their ecological significance. This study documents the presence of 35 species of Coccinellidae in 19 genera belonging to 10 tribes and 6 subfamilies. Seven species (mostly very rare), out of the 35 documented, are recorded for the first time in the area studied. These are Nephus (Bipunctatus) bipunctatus, N. crucifer, Scymnus (Scymnus) interruptus, S. (Parapullus) abietis, S. (Neopullus) limbatus, S. nigropictus, and S. (Pullus) suturalis. Nephus peyerimhoffi, introduced to Palestine in 1986 and later considered extirpated, is recorded from three localities in this study. The distribution of many species generally correlates with local biogeographical zones. All species recorded during the study feed on agricultural pests such as aphids and scale insects. -
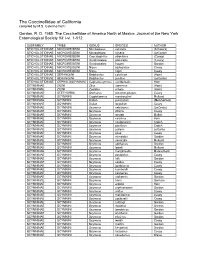
The Coccinellidae of California Compiled by M.S
The Coccinellidae of California compiled by M.S. Caterino from: Gordon, R. D. 1985. The Coccinellidae of America North of Mexico. Journal of the New York Entomological Society 93: i-vi, 1-912. SUBFAMILY TRIBE GENUS SPECIES AUTHOR STICHOLOTIDINAE MICROWEISEINI Microweisea suturalis (Schwarz) STICHOLOTIDINAE MICROWEISEINI Microweisea misella (LeConte) STICHOLOTIDINAE MICROWEISEINI Coccidophilus atronitens (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea planiceps (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea hageni Gordon STICHOLOTIDINAE MICROWEISEINI Nipus biplagiatus Casey STICHOLOTIDINAE MICROWEISEINI Nipus niger Casey STICHOLOTIDINAE SERANGIINI Delphastus catalinae (Horn) STICHOLOTIDINAE SERANGIINI Delphastus pusillus (LeConte) STICHOLOTIDINAE CEPHALOSCYMNINI Cephaloscymnus occidentalis Horn SCYMNINAE ZILINI Zilus aterrimus (Horn) SCYMNINAE ZILINI Zagloba ornata (Horn) SCYMNINAE STETHORINI Stethorus punctum picipes Casey SCYMNINAE SCYMNINI Cryptolaemus montrouzieri Mulsant SCYMNINAE SCYMNINI Didion punctatum (Melsheimer) SCYMNINAE SCYMNINI Didion longulum Casey SCYMNINAE SCYMNINI Scymnus nebulosus (LeConte) SCYMNINAE SCYMNINI Scymnus dificilis Casey SCYMNINAE SCYMNINI Scymnus fenderi Malkin SCYMNINAE SCYMNINI Scymnus caurinus Horn SCYMNINAE SCYMNINI Scymnus coniferarum Crotch SCYMNINAE SCYMNINI Scymnus pacificus Crotch SCYMNINAE SCYMNINI Scymnus pallens LeConte SCYMNINAE SCYMNINI Scymnus gilae Casey SCYMNINAE SCYMNINI Scymnus mimoides Gordon SCYMNINAE SCYMNINI Scymnus cervicalis Mulsant SCYMNINAE SCYMNINI Scymnus apithanus Gordon -

Insects of the Idaho National Laboratory: a Compilation and Review
Insects of the Idaho National Laboratory: A Compilation and Review Nancy Hampton Abstract—Large tracts of important sagebrush (Artemisia L.) Major portions of the INL have been burned by wildfires habitat in southeastern Idaho, including thousands of acres at the over the past several years, and restoration and recovery of Idaho National Laboratory (INL), continue to be lost and degraded sagebrush habitat are current topics of investigation (Ander- through wildland fire and other disturbances. The roles of most son and Patrick 2000; Blew 2000). Most restoration projects, insects in sagebrush ecosystems are not well understood, and the including those at the INL, are focused on the reestablish- effects of habitat loss and alteration on their populations and ment of vegetation communities (Anderson and Shumar communities have not been well studied. Although a comprehen- 1989; Williams 1997). Insects also have important roles in sive survey of insects at the INL has not been performed, smaller restored communities (Williams 1997) and show promise as scale studies have been concentrated in sagebrush and associated indicators of restoration success in shrub-steppe (Karr and communities at the site. Here, I compile a taxonomic inventory of Kimberling 2003; Kimberling and others 2001) and other insects identified in these studies. The baseline inventory of more habitats (Jansen 1997; Williams 1997). than 1,240 species, representing 747 genera in 212 families, can be The purpose of this paper is to present a taxonomic list of used to build models of insect diversity in natural and restored insects identified by researchers studying cold desert com- sagebrush habitats. munities at the INL. -

Pleistocene) Insect Assemblages from Illinois Kristine D
University of North Dakota UND Scholarly Commons Theses and Dissertations Theses, Dissertations, and Senior Projects 1985 Middle and Late Wisconsinan (Pleistocene) insect assemblages from Illinois Kristine D. Carter University of North Dakota Follow this and additional works at: https://commons.und.edu/theses Part of the Geology Commons Recommended Citation Carter, Kristine D., "Middle and Late Wisconsinan (Pleistocene) insect assemblages from Illinois" (1985). Theses and Dissertations. 52. https://commons.und.edu/theses/52 This Thesis is brought to you for free and open access by the Theses, Dissertations, and Senior Projects at UND Scholarly Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of UND Scholarly Commons. For more information, please contact [email protected]. MIDDLE AND LATE WISCONSINAN (PLEISTOCENE) INSECT ASSEMBLAGES FROM ILLINOIS by Kristine D. Carter Bachelor of Science, North Dakota State University, 1981 B~chelor of Arts, Moorhead State University, 1978 A thesis submitted to the graduate faculty of the University of North Dakota in partial fulfillment of the requirements for the degree of Master of Arts Grand Forks, North Dakota May 1985 I" This thesis submitted by Kristine D. Carter in partial fulfillment of the requirements for the degree of Master of Arts from the University of North Dakota is hereby approved by the Faculty Advisory Committee under whom the work was done. This thesis meets the standards for appearance and conforms to the style and format requirements of the Graduate School of the University of North Dakota, and is hereby approved. Dean the Graduate School 55297:1 l. -

History of the Biodiversity of Ladybirds (Coccinellidae) at the Black Sea Coast of the Russian Caucasus in the Last 120 Years—
insects Article History of the Biodiversity of Ladybirds (Coccinellidae) at the Black Sea Coast of the Russian Caucasus in the Last 120 Years—Does the Landscape Transformation and Establishment of Harmonia axyridis Have an Impact? Andrzej O. Bie ´nkowskiand Marina J. Orlova-Bienkowskaja * A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, 119071 Moscow, Russia; [email protected] * Correspondence: [email protected] Received: 27 October 2020; Accepted: 21 November 2020; Published: 23 November 2020 Simple Summary: Studies of the history of regional insect fauna are important for understanding the changes in ecosystems and are therefore crucial for conservation decisions. The harlequin ladybird is a global invader that causes the decline of native ladybirds in some countries. Therefore, it is advisable to monitor the ladybird fauna in regions recently occupied by this species. We analyzed the dynamics of the fauna at the main sea resort of Russia over a period of 120 years to determine the following: (1) how the ladybird biodiversity changed during the intensive landscape transformation; (2) what alien species introduced for pest control have occurred to date; and (3) what the impact is of the harlequin ladybird on the ladybird fauna. We examined specimens collected by us and 54 other collectors including specimens from old museum collections and reconstructed the history of the biodiversity like a picture from puzzle pieces. Surprisingly, landscape transformation did not cause a decrease but rather an increase in ladybird biodiversity; most of the species recorded before 1930 have occurred to date, and 23 other species have spread to the region. -
An Annotated Checklist of Ladybeetle Species (Coleoptera, Coccinellidae) of Portugal, Including the Azores and Madeira Archipelagos
ZooKeys 1053: 107–144 (2021) A peer-reviewed open-access journal doi: 10.3897/zookeys.1053.64268 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research An annotated checklist of ladybeetle species (Coleoptera, Coccinellidae) of Portugal, including the Azores and Madeira Archipelagos António Onofre Soares1, Hugo Renato Calado2, José Carlos Franco3, António Franquinho Aguiar4, Miguel M. Andrade5, Vera Zina3, Olga M.C.C. Ameixa6, Isabel Borges1, Alexandra Magro7,8 1 Centre for Ecology, Evolution and Environmental Changes and Azorean Biodiversity Group, Faculty of Sci- ences and Technology, University of the Azores, 9500-321, Ponta Delgada, Portugal 2 Azorean Biodiversity Group, Faculty of Sciences and Technology, University of the Azores, 9501-801, Ponta Delgada, Portugal 3 Centro de Estudos Florestais (CEF), Instituto Superior de Agronomia, Universidade de Lisboa, 1349-017, Lisboa, Portugal 4 Laboratório de Qualidade Agrícola, Caminho Municipal dos Caboucos, 61, 9135-372, Camacha, Madeira, Portugal 5 Rua das Virtudes, Barreiros Golden I, Bloco I, R/C B, 9000-645, Funchal, Madeira, Portugal 6 Centre for Environmental and Marine Studies and Department of Biology, University of Aveiro, Campus Universitário de Santiago, 3810-193, Aveiro, Portugal 7 Laboratoire Evolution et Diversité biologique, UMR 5174 CNRS, UPS, IRD, 118 rt de Narbonne Bt 4R1, 31062, Toulouse cedex 9, France 8 University of Toulouse – ENSFEA, 2 rt de Narbonne, Castanet-Tolosan, France Corresponding author: António Onofre Soares ([email protected]) Academic editor: J. Poorani | Received 10 February 2021 | Accepted 24 April 2021 | Published 2 August 2021 http://zoobank.org/79A20426-803E-47D6-A5F9-65C696A2E386 Citation: Soares AO, Calado HR, Franco JC, Aguiar AF, Andrade MM, Zina V, Ameixa OMCC, Borges I, Magro A (2021) An annotated checklist of ladybeetle species (Coleoptera, Coccinellidae) of Portugal, including the Azores and Madeira Archipelagos. -

Endemics Versus Newcomers: the Ladybird Beetle (Coleoptera: Coccinellidae) Fauna of Gran Canaria
insects Article Endemics Versus Newcomers: The Ladybird Beetle (Coleoptera: Coccinellidae) Fauna of Gran Canaria Jerzy Romanowski 1,* , Piotr Ceryngier 1 , Jaroslav Vetrovec˘ 2, Marta Piotrowska 3 and Karol Szawaryn 4 1 Institute of Biological Sciences, Cardinal Stefan Wyszy´nskiUniversity, Wóycickiego 1/3, 01-938 Warsaw, Poland; [email protected] 2 Buzulucká, 1105 Hradec Králové, Czech Republic; [email protected] 3 Faculty of Biology and Environmental Sciences UKSW, ul. Wóycickiego 1/3, PL-01-938 Warsaw, Poland; [email protected] 4 Museum and Institute of Zoology, Polish Academy of Sciences, Wilcza 64, 00-679 Warsaw, Poland; [email protected] * Correspondence: [email protected] Received: 18 August 2020; Accepted: 14 September 2020; Published: 18 September 2020 Simple Summary: Many plants and animals that live in the Canary Islands belong to the so-called endemic species, i.e., they do not occur outside of this particular region. Several other species have a slightly wider geographical distribution, apart from the Canaries, which also includes some islands of the nearby archipelagos, such as Madeira or the Azores, or the northwestern periphery of Africa. Here, we call such species subendemics. However, the Canary Islands have recently been colonized by a substantial number of immigrants from more or less remote areas. In this paper, based on our field survey and previously published data, we analyzed the fauna of the ladybird beetles (Coccinellidae) of Gran Canaria, one of the central islands of the archipelago. Among 42 ladybird beetle species so far recorded on this island, 17 (40%) are endemics and subendemics, and 21 (50%) probably arrived in Gran Canaria relatively recently, i.e., in the 20th and 21st century. -

VOLUME 2 Polyphaga: Scarabaeoidea Through
VOLUME 2 AMERICAN BEETLES Polyphaga: Scarabaeoidea through Curculionoidea VOLUME 2 AMERICAN BEETLES Polyphaga: Scarabaeoidea through Curculionoidea Edited by the late Ross H. Arnett, Jr., Ph.D. Michael C. Thomas, Ph.D. Paul E. Skelley, Ph.D. and J. Howard Frank, D. Phil. CRC Press Boca Raton London New York Washington, D.C. COVER FIGURES: Center - Coccinellidae, Harmonia axyridus (Palles) [Photo by Fred J. Santana]. Outer rim, clockwise from top: Ripiphoridae, Macrosiagon cruentum (Germar) [by Fred J. Santana]; Meloidae, Lytta magister Horn [by Charles L. Bellamy]; Carabidae, Rhadine exilis (Barr and Lawrence) [by James C. Cokendolpher]; Melyridae, Malachius mirandus (LeConte) [by Max E. Badgley]; Lampyridae, Microphotus angustus LeConte [by Arthur V. Evans]. Library of Congress Cataloging-in-Publication Data American beetles / edited by Ross H. Arnett and Michael C. Thomas. p. cm. Contents: v. 1. Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. Includes bibliographical references (p.). ISBN 0-8493-0954-9 (alk. paper : v. 2)) 1. Beetles—North America. I. Arnett, Ross H. II. Thomas, M. C. (Michael Charles). 1948– QL581 .A43 2002 595.76¢097—dc21 00-050809 CIP This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the authors and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage or retrieval system, without prior permission in writing from the publisher. -

Ladybeetles of Carpathian Mountains Coleoptera, Coccinellidae
Ladybeetles of Carpathian Mountains ColColeoptera,eoptera, Coccinellidae Matúš Haviar Department of Zoology Comenius University Mlynská dolina B-1 842 15 Bratislava e-mail: [email protected] Czech N Austria Hungary Poland Romania Serbia Slovakia Ukraine Subfamily Species Republic Summary Spp. CCaCCaCCaCCaCCaCCaCCaCCa 89 species in the Carpathian Mountains Coccidulinae 1 * Tetrabrachys connatus (PANZER, 1796) +++++ + ++++++ 2 Coccidula rufa (HERBST, 1783) ++++++++++ ++++ 73 species known both 3 Coccidula scutellata (HERBST, 1783) +++++++++ ++++ 4 Rhyzobius chrysomeloides (HERBST, 1792) ++++++++ ++++ in the Western and Eastern Carpathians 5 Rhyzobius litura (FABRICIUS, 1787) +++++ ++ ++++ 15 species known only in the Western Carpathians 6 Novius cruentatus (MULSANT, 1846) + + + Scymninae 1 Stethorus punctillum WEISE, 1891 ++++++++++++++++ 1 species known only in the Eastern Carpathians 2 * Clitostethus arcuatus (ROSSI, 1794) +++++ ? ++ +++ 17 species do not occur north of Carpathians 3 Scymnus abietis PAYKULL, 1798 ++++++++++ ++++ 4 Scymnus apetzi MULSANT, 1846 ++++++++++++++++ 5 Scymnus apetzoides CAPRA et FÜRSCH, 1967 +++++ 6 Scymnus ater KUGELANN, 1794 +++ ++++++ ++++ 7 Scymnus auritus THUNBERG, 1795 ++++++++++++++++ 8 Scymnus doriai CAPRA, 1924 + + 9 * Scymnus femoralis (GYLLENHAL, 1827) + ++++ ++ 10 Scymnus ferrugatus (MOLL, 1785) ++++++++++ ++++ 11 * Scymnus flagellisiphonatus FÜRSCH, 1970 + +++ ++ 12 Scymnus flavicollis REDTENBACHER,1843 ++ 13 * Scymnus fraxiniSurface FeaturesMULSANT , 1850 ++ 14 Scymnus frontalis (FABRICIUS, 1787) -

An Annotated List of Insects and Other Arthropods
This file was created by scanning the printed publication. Text errors identified by the software have been corrected; however, some errors may remain. Invertebrates of the H.J. Andrews Experimental Forest, Western Cascade Range, Oregon. V: An Annotated List of Insects and Other Arthropods Gary L Parsons Gerasimos Cassis Andrew R. Moldenke John D. Lattin Norman H. Anderson Jeffrey C. Miller Paul Hammond Timothy D. Schowalter U.S. Department of Agriculture Forest Service Pacific Northwest Research Station Portland, Oregon November 1991 Parson, Gary L.; Cassis, Gerasimos; Moldenke, Andrew R.; Lattin, John D.; Anderson, Norman H.; Miller, Jeffrey C; Hammond, Paul; Schowalter, Timothy D. 1991. Invertebrates of the H.J. Andrews Experimental Forest, western Cascade Range, Oregon. V: An annotated list of insects and other arthropods. Gen. Tech. Rep. PNW-GTR-290. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 168 p. An annotated list of species of insects and other arthropods that have been col- lected and studies on the H.J. Andrews Experimental forest, western Cascade Range, Oregon. The list includes 459 families, 2,096 genera, and 3,402 species. All species have been authoritatively identified by more than 100 specialists. In- formation is included on habitat type, functional group, plant or animal host, relative abundances, collection information, and literature references where available. There is a brief discussion of the Andrews Forest as habitat for arthropods with photo- graphs of representative habitats within the Forest. Illustrations of selected ar- thropods are included as is a bibliography. Keywords: Invertebrates, insects, H.J. Andrews Experimental forest, arthropods, annotated list, forest ecosystem, old-growth forests.