Nebraska Reportable Disease Chart
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reporting of Diseases and Conditions Regulation, Amendment, M.R. 289/2014
THE PUBLIC HEALTH ACT LOI SUR LA SANTÉ PUBLIQUE (C.C.S.M. c. P210) (c. P210 de la C.P.L.M.) Reporting of Diseases and Conditions Règlement modifiant le Règlement sur la Regulation, amendment déclaration de maladies et d'affections Regulation 289/2014 Règlement 289/2014 Registered December 23, 2014 Date d'enregistrement : le 23 décembre 2014 Manitoba Regulation 37/2009 amended Modification du R.M. 37/2009 1 The Reporting of Diseases and 1 Le présent règlement modifie le Conditions Regulation , Manitoba Règlement sur la déclaration de maladies et Regulation 37/2009, is amended by this d'affections , R.M. 37/2009. regulation. 2 Schedules A and B are replaced with 2 Les annexes A et B sont remplacées Schedules A and B to this regulation. par les annexes A et B du présent règlement. Coming into force Entrée en vigueur 3 This regulation comes into force on 3 Le présent règlement entre en vigueur January 1, 2015, or on the day it is registered le 1 er janvier 2015 ou à la date de son under The Statutes and Regulations Act , enregistrement en vertu de Loi sur les textes whichever is later. législatifs et réglementaires , si cette date est postérieure. December 19, 2014 Minister of Health/La ministre de la Santé, 19 décembre 2014 Sharon Blady 1 SCHEDULE A (Section 1) 1 The following diseases are diseases requiring contact notification in accordance with the disease-specific protocol. Common name Scientific or technical name of disease or its infectious agent Chancroid Haemophilus ducreyi Chlamydia Chlamydia trachomatis (including Lymphogranuloma venereum (LGV) serovars) Gonorrhea Neisseria gonorrhoeae HIV Human immunodeficiency virus Syphilis Treponema pallidum subspecies pallidum Tuberculosis Mycobacterium tuberculosis Mycobacterium africanum Mycobacterium canetti Mycobacterium caprae Mycobacterium microti Mycobacterium pinnipedii Mycobacterium bovis (excluding M. -

Carbapenem-Resistant Enterobacteriaceae (CRE)
Carbapenem-resistant Enterobacteriaceae (CRE) The Enterobacteriaceae include a large family of Gram-negative bacilli found in the human gastrointestinal tract. Commonly encountered species include Escherichia coli, Klebsiella spp. and Enterobacter spp. Carbapenem-resistant Enterobacteriaceae (CRE) are not susceptible to carbapenem antibiotics. They are broadly categorized based on the mechanism of their resistance as carbapenemase producers (CP-CRE) and non-carbapenemase producers. Carbapenems are broad-spectrum antibiotics typically used to treat severe health care-associated infections (HAIs) caused by highly drug-resistant bacteria. Currently available carbapenems include imipenem, meropenem, ertapenem and doripenem. Although related to the ß-lactam antibiotics, carbapenems retain antibacterial activity in the presence of most ß-lactamases, including extended-spectrum ß-lactamases (ESBLs) and extended-spectrum cephalosporinases (e.g., AmpC-type ß-lactamases). Loss of susceptibility to carbapenems is a serious problem because few safe treatment alternatives remain against such resistant bacteria. Infections caused by CRE occur most commonly among people with chronic medical conditions through use of invasive medical devices such as central venous and urinary catheters, frequent or prolonged stays in health care settings or extended courses of antibiotics. CP-CRE are most concerning and have spread rapidly across the nation and around the globe, perhaps because carbapenemases can be encoded on plasmids that are easily transferred within and among bacterial species. In December 2011, CRE bacterial isolates became reportable in Oregon. The CRE case definition has gone through major changes over the years, which is reflected in the big changes in case numbers from year to year. In 2013, the definition was non-susceptible (intermediate or resistant) to all carbapenems tested and resistant to any third generation cephalosporins tested. -

Kellie ID Emergencies.Pptx
4/24/11 ID Alert! recognizing rapidly fatal infections Susan M. Kellie, MD, MPH Professor of Medicine Division of Infectious Diseases, UNMSOM Hospital Epidemiologist UNMHSC and NMVAHCS Fever and…. Rash and altered mental status Rash Muscle pain Lymphadenopathy Hypotension Shortness of breath Recent travel Abdominal pain and diarrhea Case 1. The cross-country trucker A 30 year-old trucker driving from Oklahoma to California is hospitalized in Deming with fever and headache He is treated with broad-spectrum antibiotics, but deteriorates with obtundation, low platelet count, and a centrifugal petechial rash and is transferred to UNMH 1 4/24/11 What is your diagnosis? What is the differential diagnosis of fever and headache with petechial rash? (in the US) Tickborne rickettsioses ◦ RMSF Bacteria ◦ Neisseria meningitidis Key diagnosis in this case: “doxycycline deficiency” Key vector-borne rickettsioses treated with doxycycline: RMSF-case-fatality 5-10% ◦ Fever, nausea, vomiting, myalgia, anorexia and headache ◦ Maculopapular rash progresses to petechial after 2-4 days of fever ◦ Occasionally without rash Human granulocytotropic anaplasmosis (HGA): case-fatality<1% Human monocytotropic ehrlichiosis (HME): case fatality 2-3% 2 4/24/11 Lab clues in rickettsioses The total white blood cell (WBC) count is typicallynormal in patients with RMSF, but increased numbers of immature bands are generally observed. Thrombocytopenia, mild elevations in hepatic transaminases, and hyponatremia might be observed with RMSF whereas leukopenia -

Cutaneous Melioidosis Dermatology Section
DOI: 10.7860/JCDR/2016/18823.8463 Case Report Cutaneous Melioidosis Dermatology Section BASAVAPRABHU ACHAPPA1, DEEPAK MADI2, K. VIDYALAKSHMI3 ABSTRACT Melioidosis is an emerging infection in India. It usually presents as pneumonia. Melioidosis presenting as cutaneous lesions is uncommon. We present a case of cutaneous melioidosis from Southern India. Cutaneous melioidosis can present as an ulcer, pustule or as crusted erythematous lesions. A 22-year-old gentleman known case of diabetes mellitus was admitted in our hospital with an ulcer over the left thigh. Discharge from the ulcer grew Burkholderia pseudomallei. He was successfully treated with ceftazidime. Melioidosis must be considered in the differential diagnosis of nodular or ulcerative cutaneous lesion in a diabetic patient. Keywords: B. pseudomallei, Diabetes Mellitus, Skin ulcer CASE REPORT melioidosis is a rare entity. Cutaneous melioidosis may be primary A 22-year-old gentleman was admitted in our hospital with (presenting symptom is skin infection) or secondary (melioidosis complaints of an ulcer over the left thigh of seven days duration. at other sites in the body with incidental skin involvement) [3]. History of fever was present for four days. He also complained of There is limited published data from India documenting cutaneous pain in the thigh. The patient initially noticed a nodule on the left melioidosis. thigh which eventually progressed to form a discharging ulcer. He B. pseudomallei reside in soil and water [4]. Inoculation, inhalation was a known case of diabetes mellitus (type 1) on insulin. Clinical or ingestion of infected food or water are the modes of transmission examination revealed a 5cm× 5cm ulcer on the left thigh with [5]. -

Necrotizing Fasciitis
INFORMATION ABOUT NECROTIZING FASCIITIS • Information has been circulating on social media/media outlets of an individual who developed an infection after visiting our area. We are taking this issue seriously and are working with the Indiana Department of Health to determine if this infection was caused by bacteria such as Vibrio vulnificus or other reportable disease. Currently, we do not have any information about this individual’s illness. • Necrotizing fasciitis (many times called “flesh eating bacteria” by the media) is caused by more than one type of bacteria. Several bacteria, common in our environment can cause this condition – the most common cause of necrotizing fasciitis is Group A strep. • People do not “catch” necrotizing fasciitis; it is a complication or symptom of a bacterial infection that has not been promptly or properly treated. • Sometimes people call Vibrio vulnificus the “flesh eating bacteria.” Vibrio vulnificus is a naturally occurring bacteria found in warm salty waters such as the Gulf of Mexico and surrounding bays. Concentrations of this bacteria are higher when the water is warmer. • Necrotizing fasciitis and severe infections with Vibrio vulnificus are rare. These infections can be treated with antibiotics and sometimes require surgery to remove damaged tissue. Rapid diagnosis is the key to effective treatment and recovery. • If you are healthy with a strong immune system, your chances of developing or having complications due to this condition are extremely low. HOW TO REDUCE YOUR RISK OF EXPOSURE • The Centers for Disease Control and Prevention (CDC) encourages all people to avoid open bodies of water (such as the Gulf), pools and hot tubs with breaks in the skin. -

Staging of Necrotizing Fasciitis Based on the Evolving Cutaneous Features
ReportBlackwellOxford,IJDInternational0011-905945 UK Publishing Journal LtdLtd,of Dermatology 2006 StagingWang,Case report Wong, of necrotizing and Tay fascitis of necrotizing fasciitis based on the evolving cutaneous features Yi-Shi Wang, MBBS, MRCP, Chin-Ho Wong, MBBS, MRCS, and Yong-Kwang Tay, MBBS, FRCP From the Division of Dermatology, Changi Abstract General Hospital, Department of Plastic Background Necrotizing fasciitis is a severe soft-tissue infection characterized by a fulminant Reconstructive and Aesthetic Surgery, course and high mortality. Early recognition is difficult as the disease is often clinically Singapore General Hospital, Singapore indistinguishable from cellulitis and other soft-tissue infections early in its evolution. Our aim was Correspondence to study the manifestations of the cutaneous signs of necrotizing fasciitis as the disease evolves. Yi-Shi Wang, MBBS, MRCP Methods This was a retrospective study on patients with necrotizing fasciitis at a single Division of Dermatology institution. Their charts were reviewed to document the daily cutaneous changes from the time Changi General Hospital of presentation (day 0) through to day 4 from presentation. 2 Simei Street 3 Singapore 529889 Results Twenty-two patients were identified. At initial assessment (day 0), almost all patients E-mail: [email protected] presented with erythema, tenderness, warm skin, and swelling. Blistering occurred in 41% of patients at presentation whereas late signs such as skin crepitus, necrosis, and anesthesia Presented at the European Academy of were infrequently seen (0–5%). As time elapsed, more patients had blistering (77% had blisters Dermatology and Venereology (EADV) 14th at day 4) and eventually the late signs of necrotizing fasciitis characterized by skin crepitus, Congress, London, October 12 to 16, 2005. -

Reportable Disease Surveillance in Virginia, 2013
Reportable Disease Surveillance in Virginia, 2013 Marissa J. Levine, MD, MPH State Health Commissioner Report Production Team: Division of Surveillance and Investigation, Division of Disease Prevention, Division of Environmental Epidemiology, and Division of Immunization Virginia Department of Health Post Office Box 2448 Richmond, Virginia 23218 www.vdh.virginia.gov ACKNOWLEDGEMENT In addition to the employees of the work units listed below, the Office of Epidemiology would like to acknowledge the contributions of all those engaged in disease surveillance and control activities across the state throughout the year. We appreciate the commitment to public health of all epidemiology staff in local and district health departments and the Regional and Central Offices, as well as the conscientious work of nurses, environmental health specialists, infection preventionists, physicians, laboratory staff, and administrators. These persons report or manage disease surveillance data on an ongoing basis and diligently strive to control morbidity in Virginia. This report would not be possible without the efforts of all those who collect and follow up on morbidity reports. Divisions in the Virginia Department of Health Office of Epidemiology Disease Prevention Telephone: 804-864-7964 Environmental Epidemiology Telephone: 804-864-8182 Immunization Telephone: 804-864-8055 Surveillance and Investigation Telephone: 804-864-8141 TABLE OF CONTENTS INTRODUCTION Introduction ......................................................................................................................................1 -

RAPID IDENTIFICATION of ENTEROBACTER SPP. ISLATED from HOSPITALS in BASRAH PROVINCE by AUTOMATED SYSTEM (VITEK®2 COMPACT) Prof.Yahya A
International Journal of Micro Biology, Genetics and Monocular Biology Research Vol.2, No.2, pp.9-20, June 2016 ___Published by European Centre for Research Training and Development UK (www.eajournals.org) RAPID IDENTIFICATION OF ENTEROBACTER SPP. ISLATED FROM HOSPITALS IN BASRAH PROVINCE BY AUTOMATED SYSTEM (VITEK®2 COMPACT) Prof.Yahya A. Abbas1 and Ghosoon Fadhel Radhi2 1Nassiriya Tech.Institute.Southern Tech.University 2Department of Biology, College of Science,University of Basrah,Iraq. ABSTRACT: Atotal of 676 samples were taken from various hospitals in Basrah province. These included clinical specimens(urine , blood , stool ,nasal swabs, throat swabs,ear swabs),Environmental swabs(beds,tables,ground)and milk powder of children.All isolates were subjected to the cultural,microscopical,biochemical examination and vitek2 compact used for identification of bacteria.Atotal of 153 bacterial isolates were diagnosed as Enterobacter(67 isolates E.aerogenes,65 isolates E.cloacae complex,11 isolates E.cloacae subsp cloacae ,4 isolates E.cloacae subsp dissolvens,4 isolates E.sakazakii,1 isolate E.hormaechei and 1 isolate E.asburiae) . KEYWORDS: Enterobacter, Vitek®2 Compact, Basrah Province INTRODUCTION Enterobacter belongs to domain bacteria, phylum proteobacteria, class gamma-prteobacteria, order enterobacteriales family enterobacteriaceae (Brenner etal., 2004). Enterobacter was first described by Hormaeche and Edwards(1960). Enterobacter are rod-shaped cells,motile by peritrichous flagella,some of which are encapsulated.All Enterobacter -
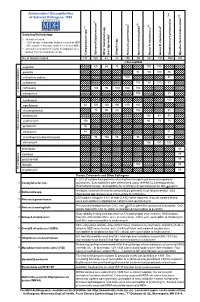
Antimicrobial Susceptibilities of Selected Pathogens, 1999
✔ Antimicrobial Susceptibilities * * † 7 of Selected Pathogens, 1999 8 e † a 2 ✔ ✔ † 3 ✔ † 4 5 6 culosis * r 1 urium spp. m L spp. Sampling Methodology 2 L † all isolates tested * ~ 20% sample of statewide isolates received at MDH spp. ~10% sample of statewide isolates received at MDH Salmonella ** all isolates tested from 7-county metropolitan area oup A streptococci ✔ oup B streptococci r isolates from a normally sterile site r Other (non-typhoidal) G Campylobacter Salmonella typhi Shigella Neisseria gonorrhoeae Neisseria meningitidis G Streptococcus pneumoni Mycobacterium tube No. of Isolates Tested 131 160 43 20 250 55 162 192 559 163 123456 123456% Susceptib123456le 123456123456 123456 123451623456 123451623456 ampicillin 1234561234566012345686 123456 15123456123456 100 100 123456123456 123451623451623451623451623456 123456 penicillin 123456123456123456123456123456 98100 100 76 123456 123456123456123456123456123456123456123456123456 123456 123451623451623451623456 123451623451623456 123456 cefuroxime sodium 123456123456123456123456 100123456123456123456 81 123456 123456123456123456123456123456123456123456123456 123456 cefotaxime 123451623451623451623451623456 100 100100 83 123456 123456123456123456123456123456 123456123456123456123456 123456 123456123456123456123456 ceftriaxone 123456 100 95 100 100 100 123451623451623451623456 -lactam antibiotics 123456123456123456123456123456 123456123456123456123456 β 123451623451623451623451623456 123451623456 123456 meropenem 123456123456123456123456123456 100 123456123456 83 123456 123456123456123456123456123456123456123456123456 -

Louisiana Morbidity Report
Louisiana Morbidity Report Office of Public Health - Infectious Disease Epidemiology Section P.O. Box 60630, New Orleans, LA 70160 - Phone: (504) 568-8313 www.dhh.louisiana.gov/LMR Infectious Disease Epidemiology Main Webpage BOBBY JINDAL KATHY KLIEBERT GOVERNOR www.infectiousdisease.dhh.louisiana.gov SECRETARY September - October, 2015 Volume 26, Number 5 Cutaneous Leishmaniasis - An Emerging Imported Infection Louisiana, 2015 Benjamin Munley, MPH; Angie Orellana, MPH; Christine Scott-Waldron, MSPH In the summer of 2015, a total of 3 cases of cutaneous leish- and the species was found to be L. panamensis, one of the 4 main maniasis, all male, were reported to the Department of Health species associated with progression to metastasized mucosal and Hospitals’ (DHH) Louisiana Office of Public Health (OPH). leishmaniasis in some instances. The first 2 cases to be reported were newly acquired, a 17-year- The third case to be reported in the summer of 2015 was from old male and his father, a 49-year-old male. Both had traveled to an Australian resident with an extensive travel history prior to Costa Rica approximately 2 months prior to their initial medical developing the skin lesion, although exact travel history could not consultation, and although they noticed bug bites after the trip, be confirmed. The case presented with a non-healing skin ulcer they did not notice any flies while traveling. It is not known less than 1 cm in diameter on his right leg. The ulcer had been where transmission of the parasite occurred while in Costa Rica, present for 18 months and had not previously been treated. -

Microbial NAD Metabolism: Lessons from Comparative Genomics
Dartmouth College Dartmouth Digital Commons Dartmouth Scholarship Faculty Work 9-2009 Microbial NAD Metabolism: Lessons from Comparative Genomics Francesca Gazzaniga Rebecca Stebbins Sheila Z. Chang Mark A. McPeek Dartmouth College Charles Brenner Carver College of Medicine Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Biochemistry Commons, Genetics and Genomics Commons, Medicine and Health Sciences Commons, and the Microbiology Commons Dartmouth Digital Commons Citation Gazzaniga, Francesca; Stebbins, Rebecca; Chang, Sheila Z.; McPeek, Mark A.; and Brenner, Charles, "Microbial NAD Metabolism: Lessons from Comparative Genomics" (2009). Dartmouth Scholarship. 1191. https://digitalcommons.dartmouth.edu/facoa/1191 This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Dartmouth Scholarship by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Sept. 2009, p. 529–541 Vol. 73, No. 3 1092-2172/09/$08.00ϩ0 doi:10.1128/MMBR.00042-08 Copyright © 2009, American Society for Microbiology. All Rights Reserved. Microbial NAD Metabolism: Lessons from Comparative Genomics Francesca Gazzaniga,1,2 Rebecca Stebbins,1,2 Sheila Z. Chang,1,2 Mark A. McPeek,2 and Charles Brenner1,3* Departments of Genetics and Biochemistry and Norris Cotton Cancer Center, Dartmouth Medical School, Lebanon, New Hampshire -

Carbapenem-Resistant Enterobcteriace Report
Laboratory-based surveillance for Carbapenem-resistant Enterobacterales (CRE) Center for Public Health Practice Oregon Public Health Division Published: August 2021 Figure1: CRE reported by Oregon laboratories, by year, 2010 – June 2021 180 160 140 120 100 80 60 40 20 0 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 Year 1 About carbapenem-resistant Enterobacterales (CRE): For more information about CRE The carbapenems are broad-spectrum antibiotics frequently used to surveillance in Oregon including treat severe infections caused by Gram-negative bacteria. the specifics of our definition, see Carbapenem resistance in the Enterobacterales order emerged as a http://public.health.oregon.gov/Di public health concern over the past decade, as few treatment options seasesConditions/DiseasesAZ/P remain for some severely ill patients. ages/disease.aspx?did=108 CRE Resistance. Carbapenem resistance emerges through various mechanisms, including impaired membrane permeability and the production of carbapenemases (enzymes that break down the carbapenems). Carbapenemase-producing CRE (CP-CRE) are associated with rapid spread and require the most aggressive infection control response; however, all CRE call for certain infection control measures, including contact precautions, and should be considered a public health and infection prevention priority. CRE Infection. CRE can cause pneumonia, bloodstream infections, surgical site infections, urinary tract infections, and other conditions, frequently affecting hospitalized patients and persons with compromised immune systems. Infections with CRE often require the use of very expensive antibiotics that may have toxic side effects. While CP-CRE have spread rapidly throughout the United States, they are still not endemic in Oregon. We hope we can delay or prevent their spread through surveillance and infection control.