Virtual Mentor American Medical Association Journal of Ethics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter Nineteen Breastfeeding Education and Support
Chapter Nineteen Breastfeeding Education and Support Chapter Nineteen – Breastfeeding Education and Support Contents Chapter Nineteen Breastfeeding Education and Support .................................................................... 19-1 Overview .............................................................................................................................................. 19-6 Introduction ..................................................................................................................................... 19-6 In This Chapter ................................................................................................................................. 19-6 Section A Breastfeeding Promotion ..................................................................................................... 19-7 Staffing ............................................................................................................................................. 19-7 Funding ............................................................................................................................................ 19-7 WIC Breastfeeding Committee ........................................................................................................ 19-7 Clinic Environment ........................................................................................................................... 19-7 Breastfeeding Messages and Resources ......................................................................................... -

Massachusetts Breastfeeding Resource Guide
Massachusetts Breastfeeding Resource Guide 2008 Edition © 254 Conant Road Weston, MA 02493 781 893-3553 Fax 781 893-8608 www.massbfc.org Massachusetts Breastfeeding Coalition Leadership Chair Board Member Melissa Bartick, MD, MS Lauren Hanley, MD Treasurer Board Member Gwen MacCaughey Marsha Walker, RN, IBCLC NABA, Lactation Associates Clerk Xena Grossman, RD, MPH Board Member Anne Merewood, MPH, IBCLC Board Member Lucia Jenkins, RN, IBCLC La Leche League of MA RI VT 10th edition (2008) Compiled and edited by Rachel Colchamiro, MPH, RD, LDN, CLC and Jan Peabody Cover artwork courtesy of Peter Kuper Funding for the printing and distribution of the Massachusetts Breastfeeding Resource Guide is provided by the Massachusetts Department of Public Health-Bureau of Family and Community Health, Nutrition Division. Permission is granted to photocopy this guide except for the three reprinted articles from the American Academy of Pediatrics and two articles from the American Academy of Family Physicians. Adapted from the Philadelphia Breastfeeding Resource Handbook. hhhhhhhhhhhhhhhhhhhhTable of Contents Organizational statements on breastfeeding…………………………………………………………….2 Breastfeeding and the Use of Human Milk, American Academy of Pediatrics…………………….6 Breastfeeding Position Paper, American Academy of Family Physicians……………………...…19 Breastfeeding initiatives………………………...……………………………………………………...…37 Breastfeeding support and services…………………………………………...………………...…....…39 La Leche League International leaders………….…………………………………………………...….40 Lactation consultants………………………………………………………………………………….…..44 -

Amy Spangler MN, RN, IBCLC
...Location Information… Conference: RAISING AWARENESS: CURRENT ISSUES IN This program is approved for 6.0 Owensboro Health Regional Hospital 1201 Pleasant Valley Road LACTATION CPEUs by the Commission on Owensboro, KY 42303 Dietetic Registration (CDR). 270-417-2000 Western Kentucky Café meeting rooms A,B,C,D Parking Lot A, Hospital entrance A, Follow signs Breastfeeding Coalition This program is approved for st to the Café mtg. rooms on 1 floor. 15th Annual Conference 4.75 L-CERPS and 1.25 R– CERPS OwensboroHealth.org by IBCLE, approval # C1551175. This program has been awarded Lodging: 7.2 Contact Hours (50 minutes Hampton Inn & Suites Downtown/Waterfront 401 W. 2nd Street per contact hour) approved by the Owensboro, KY 42301 Kentucky Board of Nursing KBN 270-685-2005 Provider # 5-0033-1-15-011. http://hamptoninn.hilton.com/en/hp/groups/ personalized/O/OWBDWHX-WKB-20150806/ index.jhtml Featuring: Deadline for block rate is July 6. Amy Spangler About the Speaker: MN, RN, IBCLC Amy Spangler earned her baccalaureate degree in nursing from Ohio State University and her master’s degree in maternal and child health from the University of Florida. She is an International Board Certified Lactation Consultant, a former president of the International Owensboro Health Lactation Consultant Association, and a former Regional Hospital chair of the United States Breastfeeding 1201 Pleasant Valley Road Committee. Amy is a member of the adjunct Owensboro, KY 42303 faculty at Emory University, College of Nursing and 270-417-2000 currently serves as President of baby gooroo. 855-417-8555 She is the author of BREASTFEEDING, A Parent’s We love babies, and we especially love Guide, BREASTFEEDING, Your guide to a healthy, happy babies. -

Breastfeeding Resources I N T H E Finger Lakes R E G I O N
Breastfeeding Resources I n t h e Finger Lakes R e g i o n Community Breastfeeding Support Resources: SPCC WIC: Breastfeeding Peer Counselor Program and Breast Pump Program. Breastfeeding Coordinator -1 888-942-6886 ext. 305 Cornell Cooperative Extension (CCE) in Wayne County. Maureen Weidman, IBCLC, RLC, Breastfeeding Classes & Breastfeeding Support 1-315-331-8415 Cornell Cooperative Extension (CCE) in Seneca County. Devra Rivkin, CLC, 1-315-539-9252 or © 1-315-651-6106 La Leche League (LLL): Mother to Mother Breastfeeding Support Groups Rochester Area:(211) 1-585-275-5151 Finger Lakes Area: 1-315-548-7980 Ontario County Public Health Maternal Child Health Nurse and CLC 1-800-299-2995 Child and Family Resources: Teresa Kennedy, CLC– 585-919-2476 ext.2506 or Natalie Ball, CLC– 315-781-1491 Breast Pumps are now covered by insurance providers due to Internet Breastfeeding Resources: the Affordable Care Act. Most providers require a prescription from your Health Care Provider. For more information, contact New York State Department of Health Breastfeeding Website a local or national durable medical supplier listed below. www.breastfeedingpartners.org Health Systems Services: 1-585-905-0583 La Leche League International’s Website 699 South Main Street, Canandaigua NY 14424 www.llli.org Southside Medical Supply: 1-585-271-7141 1815 South Clinton Ave, suite 405, Rochester Baby GooRoo Website, by Amy Spangler, RN IBCLC Nu-Life Medical Supplies: 1-585-672-5105 www.babygooroo.com 7300 Pittsford-Palmyra Rd, Fairport Westside Medical Supply : 1-585-227-8750 765 Elmgrove Rd., Rochester, NY 14624 sdrive\breastfeeding 2015\breastfeedingreasourcelist This institution is an equal opportunity provider. -

Human Milk Insights Sept 2019
September 2019 The Human Milk Insights newsletter presents the latest breastfeeding topics and clinical practice solutions, addresses coding issues challenging the lactation community, features a lactation service, and announces upcoming webinars and conferences. CONTRIBUTORS FEATURED STORIES THIS MONTH Katie McGee, RN, BSN, IBCLC Education Consultant NEWS YOU CAN USE Westchester, IL ▪ Human Milk and Legislation Maria Lennon, MSN, CNM, IBCLC ▪ Human Milk and Economics Nurse-Midwife, Perinatal Education ▪ Human Milk and Medications Consultant ▪ Human Milk Protocol Sedona, AZ. ▪ Human Milk and Organizations Irene M. Zoppi RN, MSN, IBCLC ▪ Human Milk and NICU Clinical Education Specialist ▪ Human Milk and Infant Health Medela, LLC. ▪ Human Milk and Maternal Health McHenry, IL. HUMAN MILK EDUCATION ▪ Human Milk Monthly Webinar Series ▪ Neonatal Perspectives ▪ Talking Points Flashcards ▪ Welcome CLINICAL PEARLS IN LACTATION ▪ A Serious Topic: A Mother’s Mental Health TOOLS YOU CAN USE ▪ Resources for Perinatal Mental Health SPOTLIGHT ON PRACTICE ▪ Amy K. Spangler, MN, RN, IBCLC, FILCA NEWS YOU CAN USE The Academy of Breastfeeding Medicine HUMAN MILK AND LEGISLATION released a new protocol for breastfeeding infants with cleft lip, cleft palate, or cleft lip Law requiring accommodations for and palate. breastfeeding families https://www.bfmed.org/protocols On July 25, 2019, the Fairness of Breastfeeding Mothers Act of 2019 was signed into law (Public Law No. 116-30). HUMAN MILK AND ORGANIZATIONS This Act requires that certain public buildings that contain a public restroom also USBC provide a lactation room available for use by The USBC – Affiliated Continuity of Care a member of the public. Constellation has launched a survey titled http://www.usbreastfeeding.org/fairness-act “Continuity of Care in Breastfeeding Support – Summer 2019 Survey.” The survey is aimed to develop a framework of what HUMAN MILK AND ECONOMICS continuity of care in breastfeeding support means, as well as identifying barriers and Breastfeeding Savings Calculator opportunities for improvement. -

Maintain Milk Supply
Connecticut WIC Program: Consistent Breastfeeding Education Messages: Building & Maintaining a Milk Supply Why is this important? Extending the duration of breastfeeding extends the impact of breastfeeding benefits for mom and infant. Offering guidance and support prior to delivery, within the first weeks postpartum and beyond helps women increase their duration of breastfeeding by building and maintaining a sufficient milk supply WIC’s Goal: Assist mothers in learning the skills to build and maintain a milk supply. Increase duration of breastfeeding for WIC participants. Learning Objectives: After participating in a group session or individual counseling the participant will: 1. Identify their breastfeeding goals Assess: • Willingness to breastfeed, breastfeeding goals • Social support: spouse/partner, family, friends • Medical support: WIC, Healthcare provider (ideally w/CLC or IBCLC on staff) • Community support: local BF support group, LLL 2. Understand the importance of the early postpartum period in building and maintaining a milk supply 3. Identify key steps they can take to get breastfeeding off to a good start 4. Know how to tell if their infant is getting enough breast milk 5. Learn where to go for additional support in the community to build and maintain an adequate milk supply to meet their breastfeeding goals Key Educational Messages: Prenatal BF support Affirmation: It’s good you’ve thought about how you’re planning to feed your baby. Many other moms feel overwhelmed with all the information they receive. • Put baby to breast within the first hour after delivery/ Encourage mom to practice rooming-in • Educate about colostrum and differences in onset of milk supply (DM, cesarean delivery, etc) Birth to 6 weeks postpartum Affirmation: Wow! You are doing a great job! OR Yes, a lot of mom experience that, it’s normal to feel that way. -

Pennsylvania Breastfeeding Referral Guide
PENNSYLVANIA BREASTFEEDING REFERRAL GUIDE Revised June 2020 Table of Contents Introduction .......................................................................................................................................... 5 Definitions ............................................................................................................................................ 6 Information for All Counties.................................................................................................................. 7 ADAMS................................................................................................................................................ 8 ALLEGHENY ....................................................................................................................................... 9 ARMSTRONG ................................................................................................................................... 11 BEAVER ............................................................................................................................................ 12 BEDFORD ......................................................................................................................................... 13 BERKS .............................................................................................................................................. 14 BLAIR ............................................................................................................................................... -
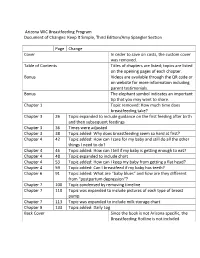
Keep It Simple, Third Edition/Amy Spangler Section
Arizona WIC Breastfeeding Program Document of Changes: Keep It Simple, Third Edition/Amy Spangler Section Page Change Cover In order to save on costs, the custom cover was removed. Table of Contents Titles of chapters are listed; topics are listed on the opening pages of each chapter. Bonus Videos are available through the QR code or on website for more information including parent testimonials. Bonus The elephant symbol indicates an important tip that you may want to share. Chapter 1 Topic removed: How much time does breastfeeding take? Chapter 3 26 Topic expanded to include guidance on the first feeding after birth and then subsequent feedings Chapter 3 36 Times were adjusted Chapter 3 38 Topic added: Why does breastfeeding seem so hard at first? Chapter 4 42 Topic added: How can I care for my baby and still do all the other things I need to do? Chapter 4 46 Topic added: How can I tell if my baby is getting enough to eat? Chapter 4 48 Topic expanded to include chart Chapter 4 53 Topic added: How can I keep my baby from getting a flat head? Chapter 4 59 Topic added: Can I breastfeed if my baby has teeth? Chapter 6 91 Topic added: What are “baby blues” and how are they different from “postpartum depression”? Chapter 7 100 Topic condensed by removing timeline Chapter 7 110 Topic was expanded to include pictures of each type of breast pump. Chapter 7 113 Topic was expanded to include milk storage chart. Chapter 9 133 Topic added: Daily Log Back Cover Since the book is not Arizona specific, the Breastfeeding Hotline is not included. -

The CDC Guide to Breastfeeding Interventions
U.S. Department of Health and Human Services Katherine R. Shealy, MPH, IBCLC, RLC Ruowei Li, MD, PhD Sandra Benton-Davis, RD, LD Laurence M. Grummer-Strawn, PhD U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion Division of Nutrition and Physical Activity Acknowledgments We gratefully acknowledge and thank all contributors and reviewers of The CDC Guide to Breastfeeding Interventions. The efforts of Jane Heinig, PhD, IBCLC, RLC, Deborah Galuska, PhD, Diana Toomer, Barbara Latham, RD, LD, Carol MacGowan, MPH, RD, LD, Robin Hamre, MPH, RD, and members of the CDC Obesity Team helped make this document possible. Publication Support was provided by Palladian Partners, Inc., under Contract No. 200-980-0415 for the National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Photographs contained in this guide were purchased solely for educational purposes and may not be reproduced for commercial use. Recommended Citation Shealy KR, Li R, Benton-Davis S, Grummer-Strawn LM. The CDC Guide to Breastfeeding Interventions. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2005. For more information or to download this document or sections of this document, please visit http://www.cdc.gov/breastfeeding To request additional copies of this document, please email your request to [email protected] or write to us at the following address and request The CDC Guide to Breastfeeding Interventions: Maternal and Child Nutrition Branch, Division of Nutrition and Physical Activity National Center for Chronic Disease Prevention and Health Promotion Centers for Disease Control and Prevention 4770 Buford Highway, NE Mailstop K–25 Atlanta, Georgia 30341-3717 Contents Introduction Introduction . -

Baby and You Getting Ready for Motherhood & Baby
Baby and You Getting Ready for Motherhood & Baby Battle Creek Adjusting to Pregnancy Changes Pregnancy is a major life change. Your entire life will have new direction and focus after the birth of your baby. For most of us, the joys of pregnancy and parenthood make for many ups and downs. Things affected by pregnancy and birth include: Table of Contents – 1st Trimester • Your body • Your relationship with others • Your work, school, and daily schedules Adjusting to Pregnancy ........................ 3 • Your nutritional needs • Your moods - up one minute, down the next Nutrition ................................................ 4 Things to Do Preparation for Childbirth .................... 5 Things that you can do to help you enjoy being pregnant are: • Read the handouts given to you by your nurse, midwife or doctor • Ask your nurse how you can sign up for classes • Write down your questions and bring them with you to your appointments Notes • Get plenty of sleep • Eat a healthy diet • See your doctor, nurse or midwife regularly Talking to your friends and family is important. Share your feelings and listen to each other. Talk to your nurse, midwife or doctor if you are having a hard time coping with your pregnancy due to the following problems. • Emotional • Physical • Money When to Call Your Nurse, Midwife or Doctor While most women have a healthy pregnancy, things can occur that need the attention of your medical professional. We want you to tell us if you notice any of the following: • Vaginal bleeding • Changes in your vision • Abdomen tightening or cramping • Swelling of your face or fingers • Backache that comes and goes • Burning or pain on urination • Severe abdominal pain • Fever or chills • Any fluid coming from the vagina (birth canal) • Nausea or vomiting for more than 24 hours • Severe headache that won’t go away • Your baby isn’t moving normally (after 5 months) For More Information As a part of the Baby & You program, you received a coupon for a free copy of the book What to Expect When You’re Expecting. -

Breastfeeding
Washington WIC – Certifier Competency Training Worksheet 12. Breastfeeding Competency Certifier is able to: Training Requirements Promote and support View the DOH STATE WIC Breastfeeding course and The Learning Center (TLC) breastfeeding as the complete the post-test with 80% or higher score. • DOH STATE WIC Breastfeeding Curriculum normal way to feed a Assess real and perceived barriers to breastfeeding. baby. WIC Manual - Volume 1 Assist adult participant to overcome barriers. • Chapter 15-Breastfeeding Be familiar with Recognize health and lifestyle contraindications to agency breast pump breastfeeding. Breastfeeding Resources Spreadsheet policies. • Breastfeeding Resources Spreadsheet (Excel) Apply knowledge of anatomy and physiology in the assessment of normal breastfeeding. Washington WIC Breastfeeding and Baby Analyze breastfeeding problems using evidence- Behavior Materials (WAWIC webpage): based information as the standard. • Healthy Sleep for you and baby Evaluate the impact of returning to work or school • Understanding Baby’s Cues and the participant’s breastfeeding goals. • Why babies cry? Answer common questions about breastfeeding. • Crib Card Identify participants who need a breast pump. Staff Resource Refer participants who need a breast pump to staff • Breastfeed right from the start! who issue breast pumps. Participant Nutrition Education Handouts These are available from the Fulfillment Center: • 10 Tips for Dads to Help Support Breastfeeding Moms • How Does Formula Compare to Breastmilk? • Baby’s First Month Nut Ed -

Keep It Simple! Make It Real 2 Questions…
Keep It Simple! Make It Real Amy Spangler, MN, RN, IBCLC How long will you 2 Questions… breastfeed? § How long will you § Will not breastfeed? § Unsure § Baby decides § Why have you stopped? § Morning and night only § 6 weeks § 12 weeks § 4-6 months § 1 year What does the Why have you stopped? evidence tell us? § Insufficient milk § Insufficient milk § Pain - Perceived insufficient milk is nothing new § Return to work / school - Reflects a lack of _________ § Baby prefers bottle § Baby has teeth § Pregnant 1 What does the evidence tell us? § Pain __ out of 5 mothers report pain. What does the evidence tell us? § Return to work / school ____ percent of women with children under 3 years of age work outside the home. What does the evidence tell us? § Baby prefers bottle There is ___ evidence for nipple preference / confusion. 2 What does the evidence tell us? § Baby has teeth Babies are unlikely to ___ the ___ that feeds them! What does the evidence tell us? § Pregnant women ___ breastfeed. § Breastfeeding women ___ get pregnant. If breastfeeding is normal… Why is it so hard? 3 Unrealistic expectations How do normal breastfed babies behave? What parents NEED to know or NOT 4 FACTS • All babies poop, pee, eat, sleep, cry, and grow. 2 Questions § How often? § What color? POOP / PEE EAT 4 Questions § How often will my baby breastfeed? § How long will a feeding last? § How much breast milk does my baby take at a feeding? § When should I introduce solid foods? 5 HUNGER CUES SLEEP 3 Questions § When will my baby sleep through the night? § Do I need to wake my baby to breastfeed? § Can I sleep with my baby? Cry 3 Questions § Why? – Hungry, fussy, wet or dirty diaper, hot, cold, sick § What to do? – Feed, hold, change, unwrap, wrap, call doctor § Can’t cope? – Keep baby safe 6 3 Questions § How much weight should my Toss Out The baby lose in the first days of life? Rule Book § When should my baby be back to birth weight? § How much weight should my baby gain in the first weeks/ months of life? Rule #1 Feed the baby! 7 Rule #2 See Rule #1 Neonate vs.