Personal Cleansing Composition Comprising Highly Nourishing Oils
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essential Wholesale & Labs Carrier Oils Chart
Essential Wholesale & Labs Carrier Oils Chart This chart is based off of the virgin, unrefined versions of each carrier where applicable, depending on our website catalog. The information provided may vary depending on the carrier's source and processing and is meant for educational purposes only. Viscosity Absorbtion Comparible Subsitutions Carrier Oil/Butter Color (at room Odor Details/Attributes Rate (Based on Viscosity & Absorbotion Rate) temperature) Description: Stable vegetable butter with a neutral odor. High content of monounsaturated oleic acid and relatively high content of natural antioxidants. Offers good oxidative stability, excellent Almond Butter White to pale yellow Soft Solid Fat Neutral Odor Average cold weather stability, contains occlusive properties, and can act as a moistening agent. Aloe Butter, Illipe Butter Fatty Acid Compositon: Palmitic, Stearic, Oleic, and Linoleic Description: Made from Aloe Vera and Coconut Oil. Can be used as an emollient and contains antioxidant properties. It's high fluidiy gives it good spreadability, and it can quickly hydrate while Aloe Butter White Soft Semi-Solid Fat Neutral Odor Average being both cooling and soothing. Fatty Acid Almond Butter, Illipe Butter Compostion: Linoleic, Oleic, Palmitic, Stearic Description: Made from by combinging Aloe Vera Powder with quality soybean oil to create a Apricot Kernel Oil, Broccoli Seed Oil, Camellia Seed Oil, Evening Aloe Vera Oil Clear, off-white to yellow Free Flowing Liquid Oil Mild musky odor Fast soothing and nourishing carrier oil. Fatty Acid Primrose Oil, Grapeseed Oil, Meadowfoam Seed Oil, Safflower Compostion: Linoleic, Oleic, Palmitic, Stearic Oil, Strawberry Seed Oil Description: This oil is similar in weight to human sebum, making it extremely nouirshing to the skin. -

379 Skin Colonizer(Rebalance the Skin Microbiome)
379_SkinColonizer_Package_v1.pdf 1 2/15/17 3:12 PM #379 Skin Colonizer (Rebalance The Skin Microbiome) The Leader in Cellular Nutrition #379 Skin Colonizer (Rebalance The Skin Microbiome) The bacteria that inhabit human skin are found to serve hundreds, if not thousands, of beneficial activities including help with healing wounds, informing cell receptors to initiate cellular activities, and serving as a front-line immune system that provides protection and immune modulation regarding the myriad of pathogens that seek entrance to the body. Skin Colonizer is an innovative breakthrough in product formulation by presenting the body with truly viable cultures. t INDICATIONS • Kukui Oil - A natural moisturizer used by the Hawaiians for hundreds of years, and is touted to support normal wound • Support normal skin microbiome healing. A rich source of antioxidants. • Skin comfort regarding occasional rashes • Marula Oil - A source of Omega-6 and Omega-9 fatty • Skin comfort regarding occasional itches acids famous for nourishing the skin. Cited to help normal ™ tSupplement INGREDIENTS Facts Servings per container: About 30 • Occasional diaper rash comfort preservation of transepidermal water migration—thus an Serving size: 1 mL excellent moisturizer. Skin Microbiome Calories 8.1 • Comfort regarding exanthems Amount per serving % Daily Value • Perilla Oil - Contains a special, essential fatty acid, n-3 linolenic colonizer provides a Total Fat 0.9 g 1% • Support normal gut-skin-brain microbial axis Vitamin E (d-alpha-Tocopheryl Acetate) 9 IU 30% acid that plays a major role in regulating normal inflammation C unique combination of Vitamin D 250 IU 63% • Support of skin microbiome species diversity processes in the skin. -

The Soap Making Companion a Guide for Beginners 2Nd Edition
The Soap Making Companion A Guide for Beginners 2nd Edition The companion guidebook to the Thermal Mermaid Artisan Soap Makers Course By Thermal Mermaid & Jennifer Tynan Copyright 2020 by SpeckledEggPublishing All rights reserved. This book, or parts within may not be reproduced without permission from the publisher. Published by SpeckledEggPublishing Waterbury, CT 06706 https://speckledeggpublishing.com Contains material edited and modified from The Soap Making Companion: A Guide for Beginners, 1st Edition, by Jennifer Tynan, copyright 2018, 2015. ISBN: 9798643284789 Printed in the United States of America This publication is designed to provide accurate information in respect to the material presented. It is sold with the understanding that the recipes and suggestions are personal thoughts and understanding on the artisan craft of soap making and is not engaged in professional or medical advice. No claims are made about any of the recipes regarding cures, therapies, or treatments for personal care. Soap is made for cleaning, and the recipes in this book are geared toward the artistic techniques in creating only soap. Photography by Jennifer Tynan 10 9 8 7 6 5 4 3 2 2 Table of Contents Introduction 1. 1: Soaping, Setup, & Safety Welcome to the Beginning Gather the Supplies You Need Lye Safety & First Aid How to Mix & Use Lye Properly Saponification & Lye Calculations by Hand First Simple Soap Recipe 2. Cold Process Method, Techniques & Designs White Soap Trace Lavender Flowers Gel Insulating Glycerin Rivers Soda Ash Neapolitan Clay Bar Salt Bar & Brine Drop Swirl Soap 3 Hangar Swirl In the Bowl Swirl Column Pour Peacock Swirl Lava Bubbles 3. -
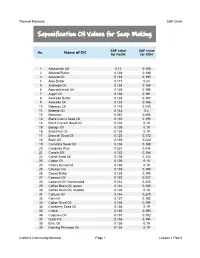
Saponification Oil Values for Soap Making
Thermal Mermaid SAP Chart Saponification Oil Values for Soap Making SAP value SAP value No. Name of Oil for NaOH for KOH 1 Abyssinian Oil 0.12 0.168 2 Almond Butter 0.134 0.188 3 Almond Oil 0.139 0.195 4 Aloe Butter 0.171 0.24 5 Andiroba Oil 0.134 0.188 6 Apricot Kernal Oil 0.139 0.195 7 Argan Oil 0.136 0.191 8 Avocado Butter 0.133 0.187 9 Avocado Oil 0.133 0.186 10 Babassu Oil 0.175 0.245 11 Baobab Oil 0.143 0.2 12 Beeswax 0.067 0.094 13 Black Cumin Seed Oil 0.139 0.195 14 Black Currant Seed Oil 0.135 0.19 15 Borage Oil 0.135 0.19 16 Brazil Nut Oil 0.135 0.19 17 Broccoli Seed Oil 0.123 0.172 18 Buriti Oil 0.159 0.223 19 Camelina Seed Oil 0.134 0.188 20 Candelila Wax 0.031 0.044 21 Canola Oil 0.133 0.186 22 Carrot Seed Oil 0.103 0.144 23 Castor Oil 0.128 0.18 24 Cherry Kernal Oil 0.135 0.19 25 Chicken Fat 0.139 0.195 26 Cocoa Butter 0.138 0.194 27 Coconut Oil 0.183 0.257 28 Coconut Oil, fractionated 0.232 0.325 29 Coffee Been Oil, green 0.132 0.185 30 Coffee Bean Oil, roasted 0.128 0.18 31 Cohune Oil 0.146 0.205 32 Corn Oil 0.137 0.192 33 Cotton Seed Oil 0.138 0.194 34 Cranberry Seed Oil 0.135 0.19 35 Crisco 0.138 0.193 36 Cupuacu Oil 0.137 0.192 37 Duck Fat 0.138 0.194 38 Emu Oil 0.135 0.19 39 Evening Primrose Oil 0.135 0.19 Crafter's Community Member Page 1 Lesson 1 Part 5 Thermal Mermaid SAP Chart SAP value SAP value No. -

Emerging Vegetable Oils in Europe
CBI Product Factsheet: Emerging vegetable oils in Europe CBI | Market Intelligence Product Factsheet Cloves in Germany | 1 Introduction The drive for innovation in the European food industry leads companies to adopt new ingredients in order to remain competitive. The introduction of new vegetable oils (emerging oils), either as ingredients or as final consumer products, is a form of innovation, differentiation and marketing used by food companies to articulate their competitive edge. On the other side of the coin, exporters of emerging oils still face a number of challenges to access the European market successfully. Whereas the Novel Food Regulation imposes marketing restrictions on a number of innovative products, some barriers to suppliers are often related to issues such as supply sufficiency and stability. At the same time, emerging oils also provide opportunities in terms of niche marketing and value adding propositions. Understanding the European market for emerging vegetable oils Innovation is high on the agenda of food manufacturers in Europe, significantly in North-Western Europe. In order to remain competitive, companies invest heavily in Research & Development (R&D), with the goal of adapting products to ever-changing consumer preferences, food health and safety requirements and introducing novelty into the food market. A large part of this strategy consists in finding new ingredient solutions. Vegetable oils are an integral part of several food products and, as such, are an interesting basis for product development. The introduction of new vegetable oils, either as ingredients or as final consumer products, is a form of innovation, differentiation and marketing used by food companies to articulate their competitive edge. -

Marula Oil Naturally Stable Emollient for a Softer You
Personal Care Consumer Care Marula Oil Naturally Stable Emollient for a Softer You INCI Name: Sclerocarya birrea Oil SAP Code#: 136070 Key Product Attributes: Background Information – ECOCERT approved The Marula tree (Sclerocarya birrea) is native to East, South and Central – Moisturizing Africa. It is one of the most highly valued indigenous wild fruit trees among – Contains essential fatty acids the black tribes of Southern Africa. The fruit, which is roughly plum-sized, – Extremely stable in cosmetic formulations contains kernels rich in oil. Lonza Personal Care has developed a high – Antioxidant properties natural antioxidant content oil derived from Marula kernels. A Social Mission Product Information Empowering Poor Communities Miligram of vitamin C present in 100ml of different beverages1: Our supplier of Marula Oil buys marula fruit harvested by rural Orange Juice 50.0 communities in the Bushbuck Ridge area in Limpopo - one of the poorest Milk 2.0 regions in South Africa. There are currently about 4000 harvesters Home-brewed beer 0.8 listed. The participants in the marula program are typically women, Marula "beer" 100.00 and this opportunity is uniquely suited to the constraints they face in Marula fruit juice 200.00 participating in economic activity. The stone within the Marula fruit contains several kernels, which are Most of the pulp is processed by hand, and about 30% is done rich in oil. Marula Oil is presently extracted by the local farmers using mechanically. The nuts from the fruit are returned to the communities for simple pressing and filtration techniques. No solvents are used, giving hand cracking. The women can do this whenever it is convenient, and in it a high purity for cosmetic applications.2 Marula Oil is rich in oleic acid, the volumes that suit the participants. -

Cosmetic Oils Assortment Henry Lamotte Oils Gmbh
Product Portfolio Cosmetics Discover our broad product range Henry Lamotte Oils GmbH is a leading supplier and producer of oils, waxes and seed flour as well as colours and flavourings – in conventional as well as in certified organic qualities. Oils and fats for the cosmetics industry ils and fats are two of the most important cosmetic ingredients and are deployed in a variety of very different external applications, for example skin care products such as creams, lotions, lipsticks, massage oils as well as hair care products such as shampoos, conditioners and hair tonics. Henry Lamotte Oils – partners to the... • cosmetics industry • pharmaceuticals industry and wholesale pharmaceuticals • food processing industries and the food wholesale and retail industry • veterinary and feed industry • chemicals industry Our services • production, contract manufacturing and bottling • worldwide purchasing and sales • quality assurance and in-house laboratories • quality management • product development, customer-specific recipes • EU certified organic operation (DE-ÖKO-003) • officially licensed pharmaceutical company (GMP) • Kosher and Halal certified • BSCI-membership and continuing sustainability efforts 02.2020 Natural Special Oils – Cosmetic Ingredients: Our products are available as... • pressed • refined • hydrogenated • fractionated • conventional • certified organic ...qualities For more information do not hesitate to contact us! Oils and special ingredients Flour Açai Oil Manketti Oil Almond Flour Linseed Flour Ahiflower® Oil Marula Oil -

Kosher Certificate
COURT OF THE CHIEF RABBI BETH DIN, LONDON Dayan Ch. Ehrentreu, KASHRUT DIVISION - KLBD Emeritus Rosh Beth Din & Consultant Dayan Rabbi J. D. Conway, Director Dayan M. Gelley, Rosh Beth Din Dayan I. Binstock Dayan S. Simons KOSHER CERTIFICATE Date Page Number: 1 of 21 12.12.2019 Company Code: 3639 14th Kislev 5780 Certificate Number: 42852 The following products manufactured by International Food Science Centre A/S (IFSC A/S), Harlev J, Denmark are Kosher certified by the London Beth Din Kashrut Division (KLBD) for year round use when bearing the kosher logo and according to the Kosher status below. International Food Science Centre A/S (IFSC A/S) Cedervej 7 Harlev J 8462 Denmark Product Code Product Kosher Status Kosher Logo ACACIA SENEGAL SEED OIL Pareve non-Passover KLBD ACAI BUTTER Pareve non-Passover KLBD ACAI SEED OIL Pareve non-Passover KLBD ALFALFA OIL Pareve non-Passover KLBD ALLANBLACKIA BUTTER Pareve non-Passover KLBD ALOE BUTTER Pareve non-Passover KLBD ALOE OIL Pareve non-Passover KLBD ALOE VERA BUTTER Pareve non-Passover KLBD AMLA BUTTER Pareve non-Passover KLBD AMLA CURCUMIN BUTTER Pareve non-Passover KLBD AMLA CURCUMIN OIL Pareve non-Passover KLBD AMLA CURCUMIN/OMEGA-3 BUTTER Pareve non-Passover KLBD AMLA CURCUMIN/OMEGA-3 OIL Pareve non-Passover KLBD AMLA OIL Pareve non-Passover KLBD AMLA OIL SESAME EXTRACT Pareve non-Passover KLBD This Kosher certificate is valid until 12 December 2020 and is subject to renewal at that time. Rabbi Jeremy Conway Dayan Menachem Gelley Head Office: Kashrut Division, 305 Ballards Lane, London, N12 8GB, United Kingdom Tel: +44 (0) 20 8343 6246 Fax: +44 (0) 20 8343 6254 eMail: [email protected] Web: www.klbdkosher.org COURT OF THE CHIEF RABBI BETH DIN, LONDON Dayan Ch. -

Sustainable Sourcing of African Botanicals
WEBINAR SERIES Sustainability in Personal Care – African Botanicals LET’S BEGIN INTRODUCTIONS Presenters House Rules • All participants are on mute. • Please post all questions in the Q&A box, all questions will be answered at the end of the presentation. • A link to the product brochures will be shared with all participants. • There will be a copy of the webinar available on request. WEBINAR SERIES | SKIN-LOVING HYGIENE AND MOISTURISATION ALL RIGHTS RESERVED © 2020 | CIREBELLE 3 ABOUT CIREBELLE WEBINAR SERIES | SKIN-LOVING HYGIENE AND MOISTURISATION ALL RIGHTS RESERVED © 2020 | CIREBELLE 4 About Cirebelle Inspired by Africa, driven by Technology Cirebelle was founded in 2001 in Johannesburg, South Africa, as a manufacturer of wax-based raw materials for the Personal Care Industry Cirebelle is innovation driven, offering high- performance ingredients & specialist services At the core of Cirebelle’s values is the belief that industry can be leveraged to positively impact society As an organisation, we seek to catalyse social transformation, economic upliftment and sustainable development WEBINAR SERIES | SKIN-LOVING HYGIENE AND MOISTURISATION ALL RIGHTS RESERVED © 2020 | CIREBELLE 5 5 Products and Services Overview 01 Waxes 02 Exfoliants 03 SoftSpheres™ Applications Development Lab Guideline Formulations Prototype Development 04 African Botanicals Marketing concepts & innovation WEBINARWEBINAR SERIES SERIES – |SUSTAINABILITY SKIN-LOVING HYGIENE in PC - AFRICAN AND MOISTURISATION BOTANICALS ALL RIGHTS RESERVED © 2020 | CIREBELLE 6 Certification -

Three Major Tree Nut Oils of Southern Central Africa: Their Uses and Future As Commercial Base Oils
The International Journal of Aromatherapy (2005) 15, 177–182 The International Journal of Aromatherapy intl.elsevierhealth.com/journals/ijar Three major tree nut oils of southern central Africa: Their uses and future as commercial base oils N. Zimba, S. Wren *, A. Stucki Technical Organic Advisor, Organic Product and Market Development, P.O. Box 63433, Nairobi 00619, Kenya KEYWORDS Summary There is a pressing need for sustainability of the ecology and environ- African tree-seed oils; ment of Africa. Deforestation is taking place at an alarming rate, and this situation Sustainability; will continue to escalate unless local communities can be given a sufficiently attrac- Rural livelihoods tive and viable reason to look after their forests and animals. The development of international markets for the tree seed oils is a major step towards solving this growing dilemma. In Zambia the development of sustainable harvest systems and market outlets for the highly valued indigenous tree seed oils, along with the estab- lishment of sustainable wild harvest for ‘non-timber forest products’ to accredited international certification criteria has enable sustainable livelihoods for large num- bers of disadvantaged rural communities from indigenous plant products at the same time as helping to maintain the natural environment. c 2005 Published by Elsevier Ltd. Introduction mentioned is that they are extremely stable, even when kept under very poor conditions. From the early days of humanity, the indigenous people of Africa have learnt to use the natural re- sources available to them. This is still true to this The trees day and the Zambian trees and their products that are discussed in this article have played a signifi- The marula tree (Sclerocarya birrea) is the most cant role in the lives of many local people as widely spread in Zambia and southern Africa. -

Almond Butter, Sweet Almond Oil, Sweet Aloe
NaOH (oz.) KOH (oz.) Sodium Potassium Hydroxide Hydroxide Oil SAP (for Bar Soaps) (for Liquid Soaps) INCI Name Almond Butter, Sweet 90 - 140 0.098 0.139 Prunus amygdalus dulcis (Sweet Almond) Oil Almond Oil, Sweet 190 - 200 0.137 0.194 Prunus amygdalus dulcis (Sweet Almond) Oil Aloe Butter 220 - 260 0.176 0.247 Cocos Nucifera (Coconut) Oil and Aloe Barbadensis Leaf Extract Aloe Vera Oil 185 - 200 0.135 0.191 Apricot Kernel Butter 130 - 145 0.097 0.1361 Prunus armeniacae (Apricot) Kernel Oil Apricot Kernel Oil 185 - 195 0.135 0.19 Prunus armeniaca (Apricot) Kernel Oil Argan Oil 0.192 .136 Argania Spinosa Avocado Butter 177 - 198 0.132 0.186 Hydrogenated Persea gratissima (Avocado) Seed Oil Avocado Oil 177 - 198 0.133 0.188 Persea gratissima (Avocado) Oil Babassu Oil 245-256 0.178 0.251 Orbignya oleifera (Babassu) Seed Oil Baobab Oil 190 - 220 0.143 0.202 Adansonia digitata (Baobab) Seed Oil Beef Tallow 190 - 200 0.14 0.196 Beeswax, white 89 - 103 0.067 0.095 Beeswax Beeswax, yellow 89 - 103 0.067 0.095 Beeswax Black currant Oil 185 - 195 0.134 0.188 Ribes nigrum (Blackcurrant) Fruit Oil Borage Oil 175 - 196 0.134 0.189 Borago officinalis (Borage) Seed Oil Brazil Nut Oil 245-256 0.176 0.247 Camellia Oil 185 - 197 0.134 0.189 Camellia oleifera (Camellia) Seed Oil Candelilla Wax 43 - 65 0.038 0.053 Canola Oil 0.132 0.187 Castor Oil 175 - 187 0.127 0.179 Ricinus communis (Castor) Seed Oil Cherry Kernel Oil 182-202 0.138 0.194 Prunus avium (Cherry) Kernel Oil Cocoa Butter (deodorized)188 - 200 0.136 0.192 Theobroma cacao (Cocoa) Seed Butter -

Aid-For-Trade: Case Story
AID‐FOR‐TRADE: CASE STORY WORLD INTELLECTUAL PROPERTY ORGANIZATION (WIPO) A TREE AND TRADITIONAL KNOWLEDEGE: A RECIPE FOR DEVELOPMENT 1 AID‐FOR‐TRADE CASE STORY: WIPO A TREE AND TRADITIONAL KNOWLEDEGE: A RECIPE FOR DEVELOPMENT Country: Namibia Name: Eudafano Women’s Cooperative Type: Co-operative, Non governmental/Non profit Organization Industry: Personal Products Object of protection: Inventions, Traditional Knowledge Instrument of protection: Patents Focus: Commercialization, Partnerships, Research and Development Author: WIPO/IP Advantage Database The Marula Tree (Photo: Leandro Neumann Ciuffo) 1. Background Found throughout southern Africa, the marula tree (sclerocary birrea) has been used by humans for thousands of years, with archeological findings placing uses as early as 9000 BC. About the size of a plum with a leathery skin that is butter yellow when ripe, the fruit of the marula tree has a scented juicy white flesh that clings to a hard brown kernel. Inside the kernel are two or three seeds that are so rich in oil that a simple squeeze of the hand can release a significant amount. The flavor of marula fruit has been compared to a cocktail of guava, lychee, apple and pineapple, and harvest traditionally takes place between February and June. Marula is not only popular with humans, but also with animals; elephants have been known to travel many kilometers just to get a taste of the fruit. 1 Rich in protein and high in vitamin C, the fruit and nuts of the marula tree have allowed people to make permanent settlements throughout southern Africa. It has also been used as the base for some of the most popular alcoholic beverages in the region for generations.