Forest Structure, Diversity and Regeneration Potential Along Altitudinal Gradient in Dhanaulti of Garhwal Himalaya
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dhanaulti Travel Guide - Page 1
Dhanaulti Travel Guide - http://www.ixigo.com/travel-guide/dhanaulti page 1 Pleasant weather. Carry Light woollen, umbrella. Dhanaulti When To Max: 30.4°C Min: 23.1°C Rain: 613.7mm Tranquil silences, towering alpines Sep VISIT Pleasant weather. Carry Light woollen, and fresh mountain air define the umbrella. hill station of Dhanaulti. Max: 30.3°C Min: 21.3°C Rain: 242.3mm http://www.ixigo.com/weather-in-dhanaulti-lp-1143863 Frequented by photographers for Oct the eco-park and religious Jan Pleasant weather. Carry Light woollen. devotees for the Sarkanda Devi Famous For : Hill StatioCity Cold weather. Carry Heavy woollen. Max: 29.1°C Min: 16.6°C Rain: 41.4mm Temple, this place gives you the Max: 20.2°C Min: 6.8°C Rain: 43.6mm Nov opportunity to spend some 'me- A stone's throw away from Mussoorie, the Feb Cold weather. Carry Heavy woollen. time' away from the hustle bustle small hill station of Dhanaulti is famous for Cold weather. Carry Heavy woollen, Max: 25.8°C Min: 11.7°C Rain: 6.3mm of city life. its twin Eco-parks, Ambar and Dhara. The umbrella. most interesting fact about this eco-park is Max: 22.8°C Min: 9.4°C Rain: 56.3mm Dec that it is a joint initiative of the local youth Cold weather. Carry Heavy woollen. and the Forest Department in an attempt to Mar Max: 22.3°C Min: 7.9°C Rain: 7.6mm bring about more greenery on the barren Cold weather. Carry Heavy woollen. -

“I Love This Country”
Country Inn & Suites By Carlson Mussoorie More reasons to say, “I LOVE THIS COUNTRY” Location Country Inns & Suites by Carlson, Mussoorie, located on Convent Road, Near Library Chowk, is an idyllic detour from the hustle=-bustle of the city. With breathtaking view of the Mussoorie Ridge and the snow-capped Himalayan range, the hotel brings out an innate sense of well being to its guests. Accommodation 49 well-appointed rooms and suites, with state-of-the-art modern amenities and functional aesthetics in place, guarantee a warm, comfortable, and homely stay. Guest Rooom Facilities Wi-Fi Internet Access Complimentary Buffet Breakfast 32” LCD Satellite Television Electronic Safe Tea/Coffee Maker 24 hours In-room Dining Travel Desk Services Complimentary Newspaper Banquets & Conferencing Facilities Expansive space of 2600 sq. ft. that can accommodate upto 200 guests. Area Sit Sound Venue AC Lighting (sq. ft.) Down System Class Board Theatre U Shape Blackout Natural Room Room The Ball Room I 2600 200 100 80 90 80 The Ball Room II 1400 100 50 40 50 50 Wellness & Recreation Revive-Steeped in Bliss A sensuous harmony of time-tested wellness wisdom and skilled experts ensures that you leave the spa rejuvenated. Library-Oasis of Leisurely Reading Take a nostalgic walk down the memory lane or a racy jog down the fictional street at the hotel’s well stocked library. I N N & S U I TE S BY CARLSON® Kids’ Zone M U S S O O R I E Secure play zone for kids to channelize the energy while you enjoy a quiet coffee at the balcony overlooking it. -

Conceptual Model for the Vulnerability Assessment of Springs in the Indian Himalayas
climate Article Conceptual Model for the Vulnerability Assessment of Springs in the Indian Himalayas Denzil Daniel 1 , Aavudai Anandhi 2 and Sumit Sen 1,3,* 1 Centre of Excellence in Disaster Mitigation and Management, Indian Institute of Technology Roorkee, Roorkee 247667, India; [email protected] 2 Biological Systems Engineering Program, College of Agriculture and Food Sciences, Florida A&M University, Tallahassee, FL 32307, USA; [email protected] 3 Department of Hydrology, Indian Institute of Technology Roorkee, Roorkee 247667, India * Correspondence: [email protected]; Tel.: +91-1332-284754 Abstract: The Indian Himalayan Region is home to nearly 50 million people, more than 50% of whom are dependent on springs for their sustenance. Sustainable management of the nearly 3 million springs in the region requires a framework to identify the springs most vulnerable to change agents which can be biophysical or socio-economic, internal or external. In this study, we conceptualize vulnerability in the Indian Himalayan springs. By way of a systematic review of the published literature and synthesis of research findings, a scheme of identifying and quantifying these change agents (stressors) is presented. The stressors are then causally linked to the characteristics of the springs using indicators, and the resulting impact and responses are discussed. These components, viz., stressors, state, impact, and response, and the linkages are used in the conceptual framework to assess the vulnerability of springs. A case study adopting the proposed conceptual model is discussed Citation: Daniel, D.; Anandhi, A.; for Mathamali spring in the Western Himalayas. The conceptual model encourages quantification Sen, S. -

Initial Environmental Examination IND:Uttarakhand Emergency
Initial Environmental Examination Project Number: 47229-001 December 2014 IND: Uttarakhand Emergency Assistance Project Submitted by Uttarakhand Emergency Assistance Project (Roads & Bridges), Government of Uttarakhand, Dehardun This report has been submitted to ADB by the Program Implementation Unit, Uttarkhand Emergency Assistance Project (R&B), Government of Uttarakhand, Dehradun and is made publicly available in accordance with ADB’s public communications policy (2011). It does not necessarily reflect the views of ADB. STATUS OF COMPLIANCE WITH ADB COMMENTS AND SUGGESTIONS (ADB APPROVAL DATE 15-10-2014) SUB-PROJECT: REHABILITATION OF DAMAGED ROADS UNDER CONTRACT PACKAGES C36, C39, C41, C43, C44 and C45 ROAD SUB-PROJECT S.N. Comments/Suggestions Status of Compliance Remarks 1 We refer to the submission of initial environmental examination (lEE) report for the road sub-projects ……………….. ……………….. in Tehri district proposed for financing under UEAP. The scope includes restoration works (repairs and rehabilitation) of the damaged stretches including resurfacing; repair of pavement overlay; patch work; repairs of protection or retaining walls; repairs and restoration of culverts; and providing all necessary road furniture and roadside safety hardware for the following roads: a) Pratapnagar-Tehri Motor Road (Package No: UEAP/PWD/C36); b) Gaza-Nakot road (Package No: UEAP/PWD/C39); c) Kirtinagar-Dangdhari road (Package No: UEAP/PWD/C41); d) Tehri-Ghansali-Tilwara road (Package No: UEAP/PWD/C43); e) Chamba-Mussoorrie road (Package No: UEAP/PWD/C44); and f) Kirtinagar-Sorakhal road (Package No: UEAP/PWD/C45). 2 We note from the lEE report that the proposed works would be strictly confined within the existing ……………….. ……………….. right-of-way belonging to Public Works Department, Government of Uttarakhand; and no widening or realignment works would be undertaken under the proposed scope. -

Tehri Garhwal-CSC VLE Details
VLEs Details -Common Service Center, District-Tehri Garhwal SN District Tehsil Block VLE Name Contact Number Panchayat Address 1 TEHRI GARHWAL Ghansali bhilangana Praveen Singh Panwar 9927863108 Kontibangaon Konti Bangaon CHAMIYALA 2 TEHRI GARHWAL Narendra Nagar narendra nagar Bijendra Kumar 9927183532 \N Gaushala Road 14 Bigha 3 TEHRI GARHWAL Narendra Nagar narendra nagar Jaypal Singh 9756011442 Dhaluwalaa Rajeev Gram Dhalwala 4 TEHRI GARHWAL Narendra Nagar narendra nagar Sushil Chauhan 9627982805 Gaunsari CHAKA ROAD GAJA GAJA 5 TEHRI GARHWAL Dhanaulti jonpur Dheeraj kavi 9917138201 Myani mayani mayani 6 TEHRI GARHWAL Narendra Nagar narendra nagar Narendra Kumar 9634268366 \N KUMAR KHERA NARENDRA NAGAR 7 TEHRI GARHWAL Narendra Nagar narendra nagar Shiv Shankar 8859030746 Bugala Bugala Pawki Devi 8 TEHRI GARHWAL Devprayag kritinagar Pankaj Singh 7579041417 Khola (Kadakot) UTTARAKHAND KIRTINAGAR 9 TEHRI GARHWAL Dhanaulti jonpur ANAND SINGH 9639862245 Dhanchula village Dhanchula anand chock jaunpur 10 TEHRI GARHWAL Dhanaulti jonpur Guroovachan 9675879616 Fidogi village phidogi bhawan thatyur 11 TEHRI GARHWAL Pratapnagar jakhnidhar Devraj Singh 9410545732 Myunda 12 TEHRI GARHWAL Tehri chamba Rohit Sajwan 9557291667 Kot- KOT 13 TEHRI GARHWAL Pratapnagar pratap nagar Dinesh Singh Panwar 8394082560 Dangi Dangi Lambgaon 14 TEHRI GARHWAL Pratapnagar pratap nagar RANJAN PRASAD 9837456238 Manjaf PRATAPNAGAR ROAD THOULDHAR MANJAF 15 TEHRI GARHWAL Pratapnagar pratap nagar Arvind 8171766071 Chaundhar Main Road Vill- Chaundhar 16 TEHRI GARHWAL Pratapnagar pratap nagar Jayprakash Joshi 9760880055 Sujargaon Main Market lambgaon 17 TEHRI GARHWAL Pratapnagar pratap nagar Purushottam Joshi 8126166575 Manjaf MANJAF MANJAF 18 TEHRI GARHWAL Devprayag devprayag Munendra 8126259144 Rumdhar ROOMDHAR ROOMDHAR 19 TEHRI GARHWAL Tehri chamba KEDAR SINGH 9456334277 Chhati Gaza Road Nakot 20 TEHRI GARHWAL Pratapnagar pratap nagar Rahul Kaintura 8006776224 Deengaon DeengaonDeen Gaon Villega Lambgaon Deengaon Posta Mukhem .T. -
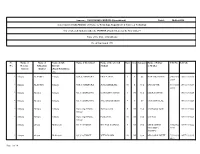
Stlist-10202016124752PM.Pdf
Annexure 12011/34/2010 INSPIRE (Uttarakhand) Dated: 20-Oct-2016 Government of India Ministry of Science & Technology, Department of Science & Technology List of Selected Students under the INSPIRE Award Scheme for the Year 2016-17 Name of the State :Uttarakhand No. of Sanctioned :190 Sr. Name of Name of Name of Sub Name of the School Name of the selected Class Sex Category Name of Father UID No Ref Code No. Revenue Education District Student or Mother District District (Block/Tehsil/Zone etc.) 1 Almora ALMORA Almora G.H.S.S.DHAURA PRIYA ARYA 9 F SC BAHADUR RAM 84852923 16UT1393218 1054 2 Almora ALMORA Almora G.H.S.S.DHAURA ARTI ADHIKARI 10 F Gen PAN SINGH 69151516 16UT1393219 2252 3 Almora Almora Almora G.I.C. BHAKUNA CHANDAN SINGH 8 M Gen HEERA SINGH 16UT1393220 4 Almora Almora Almora G.I.C. BHAKUNA SNEHA BARAKOTI 9 F SC CHANDAN LAL 16UT1393221 5 Almora Almora Almora Holy Angel Public Tanmay Joshi 8 M Gen Anil Kumar Joshi 16UT1393222 School 6 Almora Almora Almora Holy Angel Public Pankaj Pant 10 M Gen M.C.Pant 16UT1393223 School 7 Almora almora Bhikiasain G.I.C. GUMATI DEEPAK SANWAL 9 M Gen MR.RAMESH 85541924 16UT1393224 CHANDRA 5737 SANWAL 8 Almora almora Bhikiasain G.I.C. GUMATI NITYANAND 10 M Gen MR.GOPAL DUTT 73988926 16UT1393225 4677 Page 1 of 14 Sr. Name of Name of Name of Sub Name of the School Name of the selected Class Sex Category Name of Father UID No Ref Code No. Revenue Education District Student or Mother District District (Block/Tehsil/Zone etc.) 9 Almora Almora Dwarahat G.I.C. -

47229-001: Uttarakhand Emergency Assistance Project
Initial Environmental Examination Project Number: 47229-001 November 2015 IND: Uttarakhand Emergency Assistance Project Subproject : UEAP/PWD/C-79 & UEAP/PWD/C-80, Tehri District, Uttarakhand Submitted by Uttarakhand Emergency Assistance Project (Roads & Bridges), Government of Uttarakhand, Dehardun This report has been submitted to ADB by the Program Implementation Unit, Uttarkhand Emergency Assistance Project (R&B), Government of Uttarakhand, Dehradun and is made publicly available in accordance with ADB’s public communications policy (2011). It does not necessarily reflect the views of ADB. This Initial Environment Examination report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area Initial Environmental Examination October 2015 India: Uttarakhand Emergency Assistance Project Restoration & Reconstruction of: 1. Internal Motor Roads of Muni-Ki-Reti & Tapowan area (UEAP/PWD/C- 79)District Tehri, Uttarakhand 2. Internal Motor Roads of Nagar Palika Tehri (Tehri to Baurari) by BM & SDBC (3 Km. portion of SH-8 & 7.50 Km. portion urban road (UEAP/PWD/C-80) under Nagar Palika Tehri, Uttarakhand Prepared by State Disaster -

A Private Heaven Imaging Living in a Farm Surrounded by a Garden Full of Colourful Flowers and Beautiful Trees
Conceived and Developed By :- Developing and Marketing partner ABS Channel Partner ABS Promoters & Developers Head Office :- 09A-2, Dilshad Garden, Delhi-110092 Pacific Constructions & Management Futuresecure Invests Farm Site Office :- 30 KM Milestone, Dehradun, Saharapur Road, D-64, Ist Floor, Vikas Marg, Shakarpur, Delhi-110092 Village Ganeshpur, Distt Saharanpur A private heaven Imaging living in a farm surrounded by a garden full of colourful flowers and beautiful trees. Imagine staring at calm long stretches of nothingness. Wilderness of the jungle Imagine waking up to the sweet chirping of birds every morning. Classical yet contemporary It" be a place you"ll fall in love... Nature knocking on your front door Farms at Sukoon will be an awesome experience with wide variety of lifestyle. Facilities to suit your lifestyle Discover Yourself ?Hi-tech security arrangements. ?Esthetically and lavishly furnished cottage ? ?Beautiful landscaped garden Security guards. ? ?Fully secure boundary wall with entrance gate 24 hour water supply. ?Barbeque place ?100 % power backup for common facilities. ?Bonfire place ?Fully equiped beautifully landscaped club with ?Open area 90% and construction - 10% (Maximum) swimming pool. ?Serene surroundings admist wilderness of jungle ?In house grocery shop to meet your daily needs. ?In house maintenance services. Magical by Night Beautiful by Day Site map with local distances Mussoorie Kanatal Paonta Sahib Dhanaulti 19 Dehradun Sukoon – Dehradun Kms Mohand Rishikesh Rajaji National 47 Farm park Sukoon – Mussoorie Kms Haridwar Roorkee 71 Sukoon – Haridwar Kms 61 Sukoon – Rishikesh Kms Muzaffarnagar 71 Sukoon – Dhanaulti Kms 95 I am at peace Sukoon – Kanatal Kms 63 I am at rest Meerut Sukoon – Paonta Sahib Kms Modinagar I am home.... -

Tourism in Uttarakhand
TOURISM IN UTTARAKHAND: AN INTROSPECTION Dhiraj Pathak1, Indu Tiwari2, Shashi K Tiwari 3 1Assistant Professorand HOD, Faculty of Hospitality, GNA University, Phagwara (India) 2Head, Department of Botany, Govt. PG College, New Tehri, Uttarakhand (India) 3Associate Professor, Faculty of Hospitality, GNA University, Phagwara (India) I. INTRODUCTION Tourism plays an important role inemployment generation and economicgrowth of the country. It is accepted asthe potent engine for inclusive socialeconomic progress at universal levelthrough its forward and backwardlinkages and ability to create employmentin the economy. Tourism sector hasa very high employment potential withapproximately 90 jobs creation perRs. 10 lakhs of investment. There ishigh scope for profuse employmentgeneration and related activities in theform of accommodation projects, foodoriented projects, amusement parksand water sports etc.Tourism sector is one of the largestemployment generators in India andinclusive growth of the less-advantagedsections of the society and povertyeradication. The main objective of thetourism policy in India is to positiontourism as a major engine of economicgrowth and harness it’s direct andmultiplier effect on employment andpoverty eradication in a sustainablemanner by active participation of allsegments of the society Tourism plays an important role inemployment generation and economicgrowth of the country. It is accepted asthe potent engine for inclusive socialeconomic progress at universal levelthrough its forward and backwardlinkages and ability to create employmentin the economy. The present paper discusses the present status of tourism in the state of Uttarakhand and the opinion of the local population towards tourism in the state. II. OBJECTIVE To study and analyse the status of Tourism in Uttarakhand III.METHODOLOGY To assess the Status of tourism, a field based systematic survey was carried out on the basis of questionnaire in some selected tourism destinations. -

Kanatal - Rishikesh
DELHI - KANATAL - RISHIKESH Distance to Kanatal : 325 kms. (7 to 8 hrs drive) - Route : Delhi - Meerut - Muzaffarnagar - Roorkee - Haridwar - Rishikesh - Narendra Nagar - Chamba - Kanatal (On Chamba - Mussorie Road) USP of Kanatal - At 8500 ft above sea level, secluded, Awesome view of Himalayas. Clear view of Trishul, Chaukhamba and Bandarpoonch. Lots of trekking options in Kodia Jungle. Pine and Oak forest. Places to See - Kodia Jungle, Tehri Dam, Dhanolti - Surkanda devi temple, ECO Park. Road Condition - Road after Muzaffarnagar to Roorkee is bad. High traffic in Haridwar and Roorkee. Midway Break - Namaste Midway has MCD, Haldiram, Costa, Baskin and Robins is about 5 kms before Muzaffarnagar and an ideal stop over. Of course Cheetal Grand is also another option which is 12 km before Midway. Stay - 3 nights at Kanatal Club Mahindra. 1 night stay at Rishikesh GMVN Rishilok Tourist Rest House. The Journey We started by 5.30 Am. By 7.30 am we reached MCD. 10 am we were in Haridwar and by 1 pm reached Kanatal. Day - 1 Check in was smooth and post lunch we lazed around for the day... The resort is on the main Chamba - Dhanaulti road. Medium sized resort with about 35 rooms. All rooms are valley facing. Well appointed rooms with good insulation and room heating . Evenings and early morning are cold and some times windy too. The hotel is just infront of Kodia eco park. Food is pricey but members have discount and fun dining option. Monkey menace a problem. Day-2 We planned to trek Kodia Jungle. Morning trek was about 7 kms. -

Role and Importance of Leadership Skills in Hospitality Sector: an Exploratory Study of Hotels in Kumaon Region of Uttarakhand State
PSYCHOLOGY AND EDUCATION (2021) 58(2): 8522-8529 ISSN: 00333077 Role and Importance of Leadership Skills in Hospitality Sector: An Exploratory Study of Hotels in Kumaon Region of Uttarakhand State Ashish Dhyani* Department of Hospitality Management, Graphic Era Deemed to be University, Dehradun, Uttarakhand & PhD Research Scholar Uttarakhand Technical University, Dehradun, Uttarakhand Vikas Gairola*, Associate Professor, Department of Management Studies, Omkaranada Institute of Management & Technology, Rishikesh, Uttarakhand Rajat Praveen Dimri* Assistant Professor-Senior Scale, SRHU- Swami Ram Himalayan University, Jollygrant,Dehradun, Uttarakhand ABSTRACT: This paper intends to analyse the role and importance of Soft skills like leadership skills in the hospitality sector in Kumaon region of Uttarakhand state. The purpose is to explore the role of soft skills viz. leadership and team-building skills emphasized by the hospitality professionals. The study is based on the respondents’ inclination embarking upon the Leadership and team-building Skills in the hospitality sector for effective and efficient operations. To answer the research question, the study was carried out in the hotels of Kumaon region of Uttarakhand state during a two months period of December 2019 and January 2020. The responses were recorded in a questionnaire on a Likert scale of 5. The results of the research indicated that the requirement of the leadership skills along with team-building skills, is significant for managers and to be and efficient leaders in all levels of management. The findings can suggest the future implementation of the study can be helpful in operating the hotel as a unit in more effective and collaborative manner and hence contributing significantly towards the revenue generation. -

47229-001: Restoration Work of Pauri District, Uttarakhand
Initial Environmental Examination Project Number: 47229-001 December 2014 IND: Uttarakhand Emergency Assistance Project Submitted by Uttarakhand Emergency Assistance Project (Roads & Bridges), Government of Uttarakhand, Dehardun This report has been submitted to ADB by the Program Implementation Unit, Uttarkhand Emergency Assistance Project (R&B), Government of Uttarakhand, Dehradun and is made publicly available in accordance with ADB’s public communications policy (2011). It does not necessarily reflect the views of ADB. Initial Environmental Examination September 2014 India: Uttarakhand Emergency Assistance Project Restoration work of Pauri district, Uttarakhand 1. Buwakhal-Pauri-Devprayag-Gaja-Jajal (Package no:UEAP/PWD/c35) 2. Kanda-Sumari-Bugani Road (Package no: UEAP/ PWD/c40) 3. Pathradakhal Gaitichera (Package no: UEAP/PWD/c42) Prepared by State Disaster Management Authority, Government of Uttarakhand, for the Asian Development Bank. ABBREVIATIONS ADB - Asian Development Bank ASI - Archeological Survey of India BOQ - Bill of Quantity CTE - Consent to Establish CTO - Consent to Operate DFO - Divisional Forest Officer DSC - Design and Supervision Consultancy DOT - Department of Tourism CPCB - Central Pollution Control Board EA - Executing Agency EAC - Expert Appraisal Committee EARF - Environment Assessment and Review Framework EC - Environmental Clearance EIA - Environmental Impact Assessment EMMP - Environment Management and Monitoring Plan EMP - Environment Management Plan GMVN Gharwal Mandal Vikas Nigam GoI - Government of