New Strategies for the Post-Marketing Monitoring of Medical Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table of Contents
Table of Contents Welcome Message from the General Chair.......................................................... 2 M&N 2019 Organizers .......................................................................................... 3 Sponsors .............................................................................................................. 6 Patrons ................................................................................................................. 7 Exhibitors ............................................................................................................ 10 Monday, July 8 ....................................................................................................12 Tuesday, July 9 ................................................... ................................................19 Wednesday, July 10 ........................................................................................... 26 1 Welcome Message from the General Co-Chairs Dear colleagues and friends, On behalf of the entire Conference Committee, we are pleased to welcome you to the 5th IEEE International Symposium on Measurements and Networking (M&N 2019), which is held in Catania and hosted in Museo Diocesano in the heart of the city. The Symposium is mainly promoted by the IEEE IMS TC-37 Measurements and Networking, the IEEE IM Italy Chapter and by the IEEE Italy Section Systems Council Chapter. IEEE M&N is a privileged forum for the discussion of current and emerging trends on measurements, communications, computer science, -

67Th Economic Policy Panel
67th Economic Policy Panel Hosted by the Swiss National Bank Venue: Great Guild Hall, 2nd Floor, Zunfthaus zur Zimmerleuten Limmatquai 40, 8001 Zurich, Switzerland 12-13 April 2018 Programme Each session is 70 minutes in duration Author: 25 mins | Discussant: 15 mins (each) | Panel discussion: 15 mins * indicates presenting author Thursday 12 April 14:00 Registration and coffee on arrival 14:30 – 14:45 Opening Remarks Mr Thomas Moser, Alternate Member of the Governing Board, Swiss National Bank 14:45 – 15:55 Can Education Compensate the Effect of Population Aging on Macroeconomic Performance? Evidence from Panel Data Rainer Kotschy (LMU Munich) * Uwe Sunde (LMU Munich) Discussants: Francesco Drago (University of Messina) Pietro Biroli (University of Zurich) 15:55 – 17:05 Monetary Policy and Bank Profitability in a Low Interest Rate Environment * Carlo Altavilla (European Central Bank) Miguel Boucinha (European Central Bank) José-Luis Peydró (ICREA-UPF, CREI & BGSE) Discussants: Ralph De Haas (EBRD) Vasso Ioannidou (University of Lancaster) 17:05 – 17:20 coffee break 17:20 – 18:30 The Walking Dead?: Zombie Firms and Productivity Performance in OECD Countries Müge Adalet McGowan (OECD) Dan Andrews (OECD) * Valentine Millot (OECD) Discussants: Martin Brown (University of St.Gallen) Elena Carletti (Bocconi University) 20:00 Conference dinner at The Ballroom of the Savoy Hotel Baur en Ville Address: Poststrasse 12, 8001 Zurich Welcome address by Ms Andréa M. Maechler, Member of the Governing Board, Swiss National Bank Friday 13 April 08:30 – 09:40 Where Do People Get Their News? * Patrick Kennedy (Columbia University) Andrea Prat (Columbia University) Discussants: Roberto Galbiati (Sciences Po) David Hémous (University of Zurich) 09:40 – 10:50 Populism and Civil Society * Tito Boeri (Bocconi University) Prachi Mishra (IMF) Chris Papageorgiou (IMF) Antonio Spilimbergo (IMF) Discussants: Christina Gathmann (Heidelberg University) 10:50 – 11:10 Coffee break 11:10 – 12:20 The Gains from Economic Integration David Comerford (University of Strathclyde) * José V. -

Masters Erasmus Mundus Coordonnés Par Ou Associant Un EESR Français
Les Masters conjoints « Erasmus Mundus » Masters conjoints « Erasmus Mundus » coordonnés par un établissement français ou associant au moins un établissement français Liste complète des Masters conjoints Erasmus Mundus : http://eacea.ec.europa.eu/erasmus_mundus/results_compendia/selected_projects_action_1_master_courses_en.php *Master n’offrant pas de bourses Erasmus Mundus *ACES - Joint Masters Degree in Aquaculture, Environment and Society (cursus en 2 ans) UK-University of the Highlands and Islands LBG FR- Université de Nantes GR- University of Crete http://www.sams.ac.uk/erasmus-master-aquaculture ADVANCES - MA Advanced Development in Social Work (cursus en 2 ans) UK-UNIVERSITY OF LINCOLN, United Kingdom DE-AALBORG UNIVERSITET - AALBORG UNIVERSITY FR-UNIVERSITÉ PARIS OUEST NANTERRE LA DÉFENSE PO-UNIWERSYTET WARSZAWSKI PT-UNIVERSIDADE TECNICA DE LISBOA www.socialworkadvances.org AMASE - Joint European Master Programme in Advanced Materials Science and Engineering (cursus en 2 ans) DE – Saarland University ES – Polytechnic University of Catalonia FR – Institut National Polytechnique de Lorraine SE – Lulea University of Technology http://www.amase-master.net ASC - Advanced Spectroscopy in Chemistry Master's Course FR – Université des Sciences et Technologies de Lille – Lille 1 DE - University Leipzig IT - Alma Mater Studiorum - University of Bologna PL - Jagiellonian University FI - University of Helsinki http://www.master-asc.org Août 2016 Page 1 ATOSIM - Atomic Scale Modelling of Physical, Chemical and Bio-molecular Systems (cursus -

MARILENA BAZZANO Educational Activity 2020-2021
CURRICULUM VITAE – MARILENA BAZZANO Educational Activity 2020-2021 - Lecturer in Veterinary Internal Medicine Practical Activities (1 CFU), School of Veterinary Medicine, University of Camerino (UNICAM). 2020-2021 – Lecturer in Veterinary Obstetric Techniques (5 CFU), Post-Graduate School of Animal Health, Breeding and Production, University of Camerino (UNICAM) 2020-2021 – Lecturer in Control of Diseases in Livestock Production Systems (5 CFU), Animal Production Science and Valorization of Animal Derived Food, University of Camerino (UNICAM). 2019-2020 – Lecturer in Control of Diseases in Livestock Production Systems (5 CFU), Animal Production Science and Valorization of Animal Derived Food, University of Camerino (UNICAM). 2019-2020 - Lecturer in Veterinary Internal Medicine Practical Activities (3 CFU), School of Veterinary Medicine, University of Camerino (UNICAM). 2019-2020 – Lecturer in Veterinary Obstetric Techniques (10 CFU), Post-Graduate School of Animal Health, Breeding and Production, University of Camerino (UNICAM) 2018-2019 – Lecturer in Veterinary Sport Medicine (3 CFU), School of Veterinary Medicine, University of Camerino (UNICAM). 2018-2019 – Lecturer in Veterinary Internal Medicine Practical Activities (3 CFU), School of Veterinary Medicine, University of Camerino (UNICAM). 2018-2019 – Lecturer in Veterinary Obstetric Techniques (10 CFU), Post-Graduate School of Animal Health, Breeding and Production, University of Camerino (UNICAM) Work Experience and Education 2019-2020 – Tutor UNICAM for Post-Graduate grant of Dr. Marta Florence David in cooperation With the School of Veterinary Medicine of the University of Liege. 2017-2019 – Co-Supervisor of PhD course in Life and Health Sciences: One Health. XXXI Cycle. School of Advanced Studies, university of Camerino. Thesis “Study of a neW formulation of functional food for dogs With chronic renal and cardiovascular diseases”, PhD student Dr. -

PHD PROGRAMME TABLE 37TH CYCLE Section “Admission Exams” Modified on 18/06/2021
PHD PROGRAMME TABLE 37TH CYCLE Section “Admission Exams” modified on 18/06/2021 PROGRAMME’S NAME LITERARY AND PHILOLOGICAL CULTURES DURATION 3 years PROGRAMME START DATE 01/11/2021 LANGUAGE Italian MANDATORY STAY ABROAD 6 months COORDINATOR Prof. Nicola Grandi ([email protected]) CURRICULA 1. Sciences of antiquity and Sciences of books and documents 2. Italian Studies and Romance Philology RESEARCH TOPICS Detailed list at the bottom of the present document PhD POSITIONS 11 ADMISSION PROCEDURE Qualifications and research proposals evaluation Oral examination Available Positions and Scholarships Pos. n. Financial support Description Positions linked to research topics 1 PhD Scholarship Totally funded by the University of Bologna general budget 2 PhD Scholarship Totally funded by the University of Bologna general budget, with funds made available by the "Departments of Excellence" initiative 3 PhD Scholarship Totally funded by the University of Bologna general budget, with funds made available by the "Departments of Excellence" initiative 4 PhD Scholarship Totally funded by the University of Bologna general budget, with funds made available by the "Departments of Excellence" initiative 5 PhD Scholarship Totally funded by the University of Bologna general budget, with funds made available by the "Departments of Excellence" initiative 6 PhD Scholarship Funded by the University of Bologna general budget and co-funded by the Classical Philology and Italian Studies Department 7 PhD Scholarship Funded by MUR under the "Departments of General -

Print Special Issue Flyer
IMPACT FACTOR 5.076 an Open Access Journal by MDPI Graphene-Based Materials for Cancer Therapy Guest Editors: Message from the Guest Editors Prof. Dr. Daniela Iannazzo Dear Colleagues, Department of Engineering, University of Messina, Contrada Graphene-based nanomaterials such as fullerenes, carbon Di Dio, I-98166 Messina, Italy nanotubes, graphene oxide and graphene quantum dots [email protected] have shown great potential in nanomedicine and biotechnology. Their physical and chemical properties and Prof. Dr. Alessandro Pistone Department of Engineering, the presence of more reactive groups on the graphene University of Messina, Contrada surface, which allow the multimodal conjugation with Di Dio, I-98166 Messina, Italy different functional groups and biologically active [email protected] molecules, make them ideal candidates for cancer diagnosis and treatment. These nanomaterials have been conjugated with drugs and tumor-targeting ligands for a more efficient targeted delivery and have been also Deadline for manuscript submissions: investigated as imaging agents and biosensors for the 31 October 2021 identification of cancer bio-markers. “Graphene-based materials for cancer therapy” aims at collecting full papers communications and reviews that prominently demonstrate the continuous efforts in developing advanced, graphene-based nanomaterials for cancer treatment and diagnosis. Prof. Daniela Iannazzo Prof. Alessandro Pistone Guest Editors mdpi.com/si/23260 SpeciaIslsue IMPACT FACTOR 5.076 an Open Access Journal by MDPI Editor-in-Chief Message from the Editor-in-Chief Prof. Dr. Shirley Chiang Nanoscience and nanotechnology are exciting fields of Department of Physics, University research and development, with wide applications to of California Davis, One Shields electronic, optical, and magnetic devices, biology, Avenue, Davis, CA 95616-5270, USA medicine, energy, and defense. -

BOLOGNA CAMPUS ACADEMIC YEAR 2019/2020 EXCHANGE Presentation
ALMA MATER STUDIORUM UNIVERSITÀ DI BOLOGNA STUDENTS GUIDE BOLOGNA CAMPUS ACADEMIC YEAR 2019/2020 EXCHANGE Presentation Exchange Students Guide Bologna Campus A.Y. 2019/2020 Welcome to the University of Bologna! This Guide also aims to introduce you to the city Produced by This Guide is for international exchange students and its beauty, helping you to discover the places, Alma Mater Studiorum - Università di Bologna at the Bologna Campus. To make the most of your the art, events, and people that will make your DIRI - International Relations Division experience at the university and to benefit of all experience unique. Exchange Students Desk the cultural and leisure opportunities offered by Editing and grapfhic design the city of Bologna and its students community Alma Mater Studiorum - Università di Bologna it is important to learn about the rules and the ARTEC - Industrial Relations, Third Mission and Communication Division procedures to be followed and to find out about the Insitutional Communication Office services offered by the university and its territory. www.unibo.it/exchangestudents Layout Alma Mater Studiorum - Università di Bologna ARTEC - Industrial Relations, Third Mission and Communication Division The origins of the University of Bologna go way back, University of Bologna is a Multicampus university with Insitutional Communication Office and it is considered to be the oldest university in the 5 campuses: Bologna, Cesena, Forlì, Ravenna and Printed by Western world. Its history began in 1088, when law Rimini, a school of excellence, the Collegio Superiore, ACM Spa was first taught freely in the city, and became tied and an Advanced Studies Centre in Buenos Aires. -
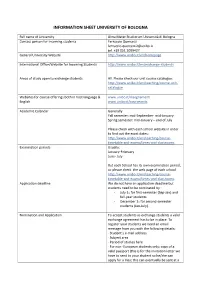
Information Sheet University of Bologna
INFORMATION SHEET UNIVERSITY OF BOLOGNA Full name of University Alma Mater Studiorum Università di Bologna Contact person for incoming students Ferruccio Quercetti [email protected] tel. +39 051 2099427 General University Website http://www.unibo.it/en/homepage International Office/Website for Incoming Students http://www.unibo.it/en/exchange-students Areas of study open to exchange students All. Please check our unit course catalogue: http://www.unibo.it/en/teaching/course-unit- catalogue Websites for course offerings both in host language & www.unibo.it/insegnamenti English www.unibo.it/courseunits Academic Calendar Generally Fall semester: mid-September- mid-January Spring semester: mid-January – end of July Please check with each school website in order to find out the exact dates: http://www.unibo.it/en/teaching/course- timetable-and-exams/times-and-classrooms Examination periods Usually: January-February June- July But each School has its own examination period, so please check the web page of each school. http://www.unibo.it/en/teaching/course- timetable-and-exams/times-and-classrooms Application deadline We do not have an application deadline but students need to be nominated by : - July 1st for first-semester (Sep-Jan) and full-year students. - December 1st for second-semester students (Jan-July). Nomination and Application To accept students as exchange students a valid exchange agreement has to be in place. To register your students we need an email message from you with the following details: · Student’s e-mail -

Curriculum Vitae ‐ Autumn 2014
Via Sarfatti 25 20136 Milano Curriculum Vitae ‐ Autumn 2014 Family name: Tagiuri Given name: Giacomo Gender: male Date of birth: (16/11/1986) Citizenship: Italian email address: [email protected] Qualifications Bocconi University Ph.D. in Legal Studies (September 2014 – 2017 expected) International Law and Economics • Full Scholarship Johns Hopkins University School of Advanced International Studies (Bologna and Washington, DC) Master of Arts in European Studies and International Economics (May 2013) • Fondazione del Monte Scholarship • Distinction in European Studies Università di Bologna Laurea Magistrale in Law (March 2011) • 110/110 magna cum laude • Erasmus Student at University of Copenhagen (January‐July 2008) Awards and Scholarships Fondazione del Monte Schoparship (Johns Hopkins University a.a. 2011/2012) Stavros Niarchos Summer Internship Funds (Johns Hopkins University, Summer 2013) University of Bologna scholarship for thesis preparation abroad (Sept.‐Dec. 2010) Bobby Abernethy Scholarship for Summer School “EU and legal reforms” (Igalo, Montenegro, Summer 2010) Research Interests International Law, Comparative Public Law, European Integration, Culture and Identity, Cultural Heritage and the Law. Publications – La dottrina Britannica in tema di diritti umani, Diritto Pubblico Comparato ed Europeo, 4/2014 – La dottrina britannica in tema di amministrazione, Diritto Pubblico Comparato ed Europeo, 1/2014. – Forging Identity: The EU and European Culture, Survival: Global Politics and Strategy, 1/2014. Article shortlisted for the International Institute of Strategic Studies (IISS)'s Palliser Essay Prize. – Letter from Italy, Survival:Global Politics and Strategy, 1/2013, pp. 197‐204. – G.Tagiuri, Romney's Italian Job, in IISS Voices, 05 November 2012 (Blog post on www.iiss.org/whats‐ new/iiss‐voices/) – Il referendum del 1975: quando i britannici decisero di rimanere nella Comunità economica europea (co‐authored with J. -

Reviewers 2020
AP&T Reviewers 2020 Highlighted reviewer denotes a top reviewer for 2020 Reviewer Last Name Reviewer First Name Reviewer Institution Reviewer Country/Region Abdel-Daim Mohamed Suez Canal University Egypt Abergel Armand Hôtel-Dieu France Abraham Neena Mayo Clinic Scottsdale United States Abraldes Juan University of Alberta Canada Afdal Nezam Beth Israel Deaconess Medical Center United States Afolabi Paul University of Soutjampton United Kingdom of Great Britain and Northern Ireland Afzal Nadeem Southampton University Hospital Trust United Kingdom of Great Britain and Northern Ireland Agardh Daniel Pediatrics Epidemiology Center United States Agarwal Banwari Royal Free Hospital United Kingdom of Great Britain and Northern Ireland Agarwal Kosh United Kingdom of Great Britain and Northern Ireland Aggarwal Rakesh Sanjay Gandhi Postgraduate Institute of Medical Sciences India Aghemo Alessio Istituto Clinico Humanitas Italy Agnholt Jørgen Aarhus University Hospital Denmark Ahmad Tariq Royal Devon and Exeter NHS Foundation Trust United Kingdom of Great Britain and Northern Ireland Ahuja Vineet All India Institute of Medical Sciences India Aithal Guruprasad University of Nottingham United Kingdom of Great Britain and Northern Ireland Alazawi William Barts and The London School of Medicine and Dentistry United Kingdom of Great Britain and Northern Ireland Alexopoulou Alexandra Greece Allez Matthieu Hôpital Saint-Louis France Allin Kristine Alpers David Washington Univ School of Medicine United States Amiot Aurélien Henri Mondor University Hospital -

Bologna Contents
Study Abroad in Bologna Contents Introduction 3 Welcome to Bologna 4 History of the University 6 The University Today 8 How to Apply 10 Tuition Fees and Living Costs 13 Living in Bologna 17 Exploring Emilia-Romagna 22 Introduction The University of Bologna is one of the most highly regarded universities in Italy, Europe, and the world. The oldest continuously running university on the planet, founded in 1088, the university and the city alike have provided countless generations of students with incredible education in one of Italy’s most historically important regions, Emilia-Romagna. It’s not all just about the history though. The University of Bologna (or UNIBO for short) consistently stands at the forefront of innovation with The University of 230 projects funded by Horizon 2020, the largest ever EU Research and Bologna is one of Innovation program, along with a further 145 funded national projects and 170 regional projects. the most highly regarded universities The university is committed to helping achieve the UN’s agenda of sustainable development goals (17 in total), teaching the best suitable in Italy, Europe, and practices to accomplish goals by 2030, with sustainability at the heart of the world. this university that has sustained teaching for close to 1,000 years. Read on to find out more about Bologna’s rich history, the history of its university, as well as UNIBO’s application process and tuition fees, what it’s like to live in the city, and discover the university multicampus across the Emilia-Romagna region. Si parte! www.TopUniversities.com How to study abroad in Bologna 3 Image from Alma Mater Studiorum - University of Bologna Welcome to Bologna Bologna is the pride and joy of northern Italy’s Emilia Romagna region, seamlessly combining the ancient and the modern. -

The Hebrew University Erasmus+ Exchange Partner Universities
The Hebrew University Erasmus+ Exchange Partner Universities Austria Germany Italy Medical University of Innsbruck (Medicine) Friedrich-Alexander-University, Erlangen-Nuremberg University of Siena (Law) – staff only University of Salzburg (Humanities) (Humanities, Social Sciences) University of Bologna (Italian Studies) University of Vienna (selected fields) Free University of Berlin (all fields) Vienna School of International Studies (European Friedrich Schiller University Jena (History, Chemistry, Lithuania Forum) Politics) Vilnius University (all fields) University of Göttingen (all fields) Belgium University of Heidelberg (all fields) Catholique de Louvain (Humanities) Humboldt University of Berlin (Humanities) Luxembourg KU Leuven (Humanities) University of Konstanz (all fields) University of Luxembourg (selected fields) Leipzig University (Humanities, Social Sciences) Croatia Ludwig Maximilian University of Munich (LMU) (all Malta University of Dubrovnik (Geography)- staff only fields) Malta University (Musicology – staff exchange Phillips-University of Marburg (all fields) only) Czech Republic Saarland University (Humanities) Masaryk University (all fields) Technical University of Munich (selected fields) Netherlands Charles University (Chemistry) University of Trier (Humanities) Leiden University (all fields) Palacky University (selected fields) Radboud University (all fields) Greece Wageningen University (Agriculture) Denmark National and Kapodistrian University of Athens Aarhus University (all fields) (Humanities - staff exchange