The Influence of Release Strategy and Migration History on Capture Rate of Oncorhynchus Mykiss in a Rotary Screw Trap Ian A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solitary Islands Marine Park Guide
Solitary Islands Marine Park Guide The NSW marine environment is one of our state’s greatest natural assets and Introduction needs to be managed for the greatest wellbeing of the community, now and into the future. The NSW Solitary Islands Marine Park was the first marine park declared in NSW. Located on the Coffs Coast, the park covers more than 70,000 hectares and 100 kilometres of coastline from the northern side of Muttonbird Island at Coffs Harbour north to Plover Island at the entrance to the Sandon River. It extends from the mean high water mark and upper tidal limits of coastal estuaries and lakes, seaward to the three nautical mile limit of NSW waters and includes the entire seabed. The Solitary Islands Marine Park (Commonwealth waters) covers 15,200 hectares on the seaward side of the NSW Solitary Islands Marine Park, out to the 50 metre depth contour. The Solitary Islands Marine Park (Commonwealth waters) is managed in partnership by the NSW Department of Primary Industries (DPI) Fisheries and Parks Australia. The NSW Solitary Islands Marine Park management rules protect the marine biodiversity of the area while supporting a wide range of social, cultural and economic values. This guide and accompanying map summarise the management rules for the NSW Solitary Islands Marine Park. For information on Solitary Islands Marine Park (Commonwealth waters) management zones please refer to the map that accompanies this guide or contact Parks Australia. 2 SOLITARY ISLANDS MARINE PARK (NSW) & SOLITARY ISLANDS MARINE PARK (COMMONWEALTH WATERS) GUIDE provides opportunities for swimming, surfing, snorkelling, Unique environmental diving, boating, fishing, walking, and panoramic ocean vistas. -

Transport for NSW Mid-North Coast Regional Boating Plan
Transport for NSW Regional Boating Plan Mid-North Coast Region February 2015 Transport for NSW 18 Lee Street Chippendale NSW 2008 Postal address: PO Box K659 Haymarket NSW 1240 Internet: www.transport.nsw.gov.au Email: [email protected] ISBN Register: 978 -1 -922030 -68 -9 © COPYRIGHT STATE OF NSW THROUGH THESECRETARY OF TRANSPORT FOR NSW 2014 Extracts from this publication may be reproduced provided the source is fully acknowledged. Report for Transport for NSW - Regional Boating Plan| i Table of contents 1. Introduction..................................................................................................................................... 4 2. Physical character of the waterways .............................................................................................. 6 2.1 Background .......................................................................................................................... 6 2.2 Bellinger and Nambucca catchments and Coffs Harbour area ........................................... 7 2.3 Macleay catchment .............................................................................................................. 9 2.4 Hastings catchment ............................................................................................................. 9 2.5 Lord Howe Island ............................................................................................................... 11 2.6 Inland waterways .............................................................................................................. -

A Community Resource
A COMMUNITY RESOURCE Acknowledgements Production of this publication has been made possible through the Australian Governments Caring for Our Country Program – Community Action Grants 2009/2010. I would like to acknowledge the assistance of other people and organisations in compiling information for the Clarence Coast and Estuary Resource Kit including CVC and NRCMA staff for their contribution of photos, maps and use of information from their projects and management plans. Pam Kenway and Debrah Novak for contributing their photos, Frances Belle Parker “Beiirrinba” image. The landowners, industries and farmers who are adopting sustainable land management practices and the people who volunteer their time towards caring for the environment. Further acknowledgements are noted throughout the resource kit. This book is based on English, N (2007) Coast and Estuary Resource Kit – A Community Resource for the Nambucca, Macleay and Hastings Valleys produced by Nambucca Valley Landcare Inc. through the National Landcare Program and Northern Rivers CMA. Aboriginal Australians Acknowledgement The Clarence estuary, coast and associated landscapes are part of the traditional lands of Aboriginal people and their nations, in particular, Yaegl people and their traditional country are acknowledged. Front Cover Image: Julie Mousley Inside Cover Image: Debrah Novak All photos are copyright © of the author Julie Mousley unless named otherwise with the image. Printed March 2011. Chapter 1: Introduction 1 Chapter 5: The importance of native vegetation 32 The -

Shorebirds of Northern NSW Final Report
© Department of Environment, Climate Change and Water NSW, 2010 The Department of Environment, Climate Change and Water NSW has compiled this document in good faith, exercising all due care and attention. The State of NSW and the Department do not accept responsibility for inaccurate or incomplete information. Readers should seek professional advice when applying this information to their specific circumstances. Department of Environment, Climate Change and Water NSW 59 – 61 Goulburn Street (PO Box A 290) Sydney South NSW 1232 Phone: 02 9995 5000 (switchboard) Phone: 131 555 (information & publications requests) Fax: 02 9995 5999 Email: [email protected] Website: www.environment.nsw.gov.au The Department of Environment, Climate Change and Water NSW is pleased to allow this material to be reproduced in whole or in part for educational and non-commercial use, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. This report is an edited version of a report by Sandpiper Ecological Surveys (Dr David Rohweder 2010) ‘Shorebird Data Audit – Northern New South Wales’, an unpublished report to the Department of Environment Climate Change and Water NSW, funded by the Northern Rivers Catchment Management Authority. This report should be cited as: Department of Environment, Climate Change and Water NSW 2010, Shorebirds of Northern New South Wales, based on a report prepared by D. Rohweder and funded by the Northern Rivers Catchment Management Authority, Department of Environment, Climate Change and Water NSW, Sydney. ISBN 978 1 74232 898 0 DECCW 2010/715 DECCW August 2010 SUMMARY Background This report is an edited version of a report by Sandpiper Ecological Surveys (Dr David Rohweder) ‘Shorebirds Data Audit – Northern New South Wales’ which was prepared for the Department of Environment, Climate Change and Water NSW and funded by the Northern Rivers Catchment Management Authority. -

Regional Pest Management Strategy 2012–17: North Coast Region
Regional Pest Management Strategy 2012–17: North Coast Region A new approach for reducing impacts on native species and park neighbours © Copyright State of NSW and Office of Environment and Heritage With the exception of photographs, the Office of Environment and Heritage (OEH) and State of NSW are pleased to allow this material to be reproduced in whole or in part for educational and non-commercial use, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. Specific permission is required for the reproduction of photographs. The New South Wales National Parks and Wildlife Service (NPWS) is part of OEH. Throughout this strategy, references to NPWS should be taken to mean NPWS carrying out functions on behalf of the Director General of the Department of Premier and Cabinet, and the Minister for the Environment. For further information contact: North Coast Region Coastal Branch National Parks and Wildlife Service Office of Environment and Heritage Department of Premier and Cabinet PO Box 361 Grafton 2460 NSW Phone: (02) 6641 1500 Report pollution and environmental incidents Environment Line: 131 555 (NSW only) or [email protected] See also www.environment.nsw.gov.au/pollution Published by: Office of Environment and Heritage 59–61 Goulburn Street, Sydney, NSW 2000 PO Box A290, Sydney South, NSW 1232 Phone: (02) 9995 5000 (switchboard) Phone: 131 555 (environment information and publications requests) Phone: 1300 361 967 (national parks, climate change and energy efficiency information and publications requests) Fax: (02) 9995 5999 TTY: (02) 9211 4723 Email: [email protected] Website: www.environment.nsw.gov.au ISBN 978 1 74293 617 8 OEH 2012/0366 August 2013 This plan may be cited as: OEH 2012, Regional Pest Management Strategy 2012–17, North Coast Region: a new approach for reducing impacts on native species and park neighbours, Office of Environment and Heritage, Sydney. -
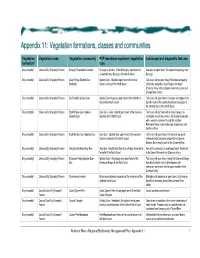
Vegetation Formations, Classes and Communities
Appendix 11: Vegetatiion formatiions, cllasses and communiities Vegetation Vegetation class Vegetation community PVP developer equivalent vegetation Landscape and diagnostic features formation* type Dry sclerophyll Clarence Dry Sclerophyll Forests Baryulgil Serpentinite Complex Eucalyptus ophitica - White Mahogany open forest on Low open or open forest. On serpentinite geology near serpentinite near Baryulgil of the North Coast Baryulgil. Dry sclerophyll Clarence Dry Sclerophyll Forests Coast Range Spotted Gum- Spotted Gum - Blackbutt open forest of the lower Tall to very tall dry open forest. Restricted and patchy Blackbutt Clarence Valley of the North Coast distribution along the Coast Range in the lower Clarence Valley, with a disjunct western occurrence in Grange State Forest. Dry sclerophyll Clarence Dry Sclerophyll Forests Dry Foothills Spotted Gum Spotted Gum dry grassy open forest of the foothills of Tall to very tall open forest. On slopes and ridges of dry the northern North Coast foothills areas of the coastal hinterland and gorges of the northern parts of the North Coast. Dry sclerophyll Clarence Dry Sclerophyll Forests Foothill Grey Gum-Ironbark- Grey Gum - Grey Ironbark open forest of the Clarence Tall to very tall dry forest with a mixed canopy. On Spotted Gum lowlands of the North Coast sandstone and siliceous soils in the Clarence lowlands with a western extension through the southern Richmond Range inland to Ewingar State Forest and the Mann River. Dry sclerophyll Clarence Dry Sclerophyll Forests Foothills Grey Gum-Spotted Gum Grey Gum - Spotted Gum open forest of the southern Tall to very tall open forest. On high and low quartz Clarence lowlands of the North Coast sediments in the southern portion of the Clarence- Moreton Basin mainly south of the Clarence River. -

Newsletter – Autumn 2017 CLARENCE ENVIRONMENT CENTRE 31 Skinner St, South Grafton 2460 Phone / Fax 66 43 1863 Email: [email protected] Website
Newsletter – Autumn 2017 CLARENCE ENVIRONMENT CENTRE 31 Skinner St, South Grafton 2460 Phone / Fax 66 43 1863 Email: [email protected] Website www.cec.org.au ____________________________________________________________________ Chaffin Creek drying up Some of those involved with the Upper Coldstream Biodiversity project will recognise the log crossing that we used to cross Chaffin Creek waterholes on the crown land at the northern end of Firth Heinz Road, home to the very healthy Giant Dragonfly population. As you can see there was no need for the 'bridge' when this picture was taken in mid February. The creek had ceased to flow, but I had never seen the waterholes so depleted. I know there was a major drought in the Clarence, and none worse affected than Pillar Valley, but I was still surprised at the dramatic drop in the level of water in these permanent waterholes. It so happens that the crown land is right next to the Pacific Highway upgrade As can be see there was no need for a 'bridge' when this picture (forest now cleared right up was taken in mid February. The creek had ceased to flow. to the crown land boundary), so I went to check on my way out, and guess what the RMS has done? 200m down-stream of the log crossing, the RMS has installed a great big pump and were drawing the last remaining water out of the billabong to suppress dust and help pack down the new road foundations. What the Hell is the 3 year's supply of water stored at Shannon Creek for? Nobody else is using it, and trucks could simply hook up to a hydrant in Tucabia without destroying the riverine habitat. -

NSW North Coast Region Irrigation Profile
NSW North Coast Region Irrigation Profile compiled by Meredith Hope for the Water Use Efficiency Advisory Unit, Dubbo The Water Use Efficiency Advisory Unit is a NSW Government joint initiative between NSW Agriculture and the Department of Sustainable Natural Resources. # The State of New South Wales NSW Agriculture (2003) This Irrigation Profile is one of a series for NSW catchments and regions. It was written and compiled by Meredith Hope, NSW Agriculture, for the Water Use Efficiency Advisory Unit, 37 Carrington Street, Dubbo, NSW, 2830. ISBN 0 7347 1373 8 (individual) ISBN 0 7347 1372 X (series) Disclaimer: This document has been prepared by the author for NSW Agriculture, for and on behalf of the State of New South Wales, in good faith on the basis of available information. While the information contained in the document has been formulated with all due care, the users of the document must obtain their own advice and conduct their own investigations and assessments of any proposals they are considering, in the light of their own individual circumstances. The document is made available on the understanding that the State of New South Wales, the author and the publisher, their respective servants and agents accept no responsibility for any person, acting on, or relying on, or upon any opinion, advice, representation, statement of information whether expressed or implied in the document, and disclaim all liability for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information contained in the document or by reason of any error, omission, defect or mis-statement (whether such error, omission or mis-statement is caused by or arises from negligence, lack of care or otherwise). -

Parks Holiday Guide
NATURe neVEr FELT SO GOoD All parks holiday guide Killen Falls, Ballina 1 MemorAblE stays in unforgETtable plAces Do everything you love to do in some of the most beautiful locations in New South Wales. You and your loved ones will That’s the beauty of the boundless always remember your stay opportunities offered by these at a Reflections Holiday Park. very special holiday destinations. After all, the sweetest memories With everything from camping last the longest. to luxury cabins, when you stay with Reflections there’s an Each of our parks have idyllic accommodation option to suit natural surroundings and every preference and budget. abundance of things to explore. You can do it all – or relax and Your only challenge is choosing do nothing at all. where to start! NSW Crown Holiday Parks Land Manager respectfully acknowledges the traditional owners of country throughout Australia and pays respect to the ongoing living cultures of Australia's First People. 2 FERRY RESERVE MASSY GREENE TERRACE RESERVE CLARKES BEACH BYRON BAY LENNOX HEAD SHAWS BAY BALLINA EVANS HEAD RED ROCK CORINDI BEACH MOONEE BEACH COPETON WATERS COFFS HARBOUR BOAMBEE CREEK RESERVE MYLESTOM URUNGA HUNGRY HEAD ARMIDALE NAMBUCCA HEADS SCOTTS HEAD LAKE KEEPIT TAMWORTH PORT MACQUARIE BONNY HILLS NORTH HAVEN TUNCURRY LAKE GLENBAWN FORSTER BEACH DUBBO SEAL ROCKS HAWKS NEST JIMMYS BEACH LAKE BURRENDONG CUDGEGONG RIVER MOOKERAWA WATERS NEWCASTLE BATHURST CENTRAL COAST SYDNEY GRABINE LAKESIDE WYANGALA WATERS WOLLONGONG KILLALEA RESERVE BURRINJUCK WATERS CANBERRA BATEMANS BAY -
Accessory Publication the Mesoveliidae, Hebridae, And
Accessory Publication The Mesoveliidae, Hebridae, and Hydrometridae of Australia (Hemiptera, Heteroptera, Gerromorpha) with a reanalysis of the phylogeny of semiaquatic bugs Nils Møller AndersenA and Tom A. WeirB AZoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark. BCSIRO Entomology, GPO Box 1700, Canberra ACT 2601, Australia. Email: [email protected] Abstract The semiaquatic bugs (Hemiptera-Heteroptera, infraorder Gerromorpha), comprising water striders and their allies, are familiar inhabitants of water surfaces in all continents. Currently, the world fauna has more than 1,900 described species classified in eight families and 165 genera.A phylogenetic analysis using maximum parsimony was performed on a dataset comprising 56 morphological characters scored for 24 examplar genera covering all families and subfamilies of Gerromorpha. The phylogenetic relationships found concur with those presented by Andersen (1982) except that the relationships between some subfamilies of Veliidae andGerridae are unresolved. The Australian fauna of Gerromorpha comprises six families, 30 genera, and 123 species. One third of the genera and more than 80% of the species are endemic to Australia. Previously, we have covered all Australian species of the families Gerridae, Hermatobatidae, and Veliidae. The present paper deals with the families Hebridae, Hydrometridae, and Mesoveliidae. We offer redescriptions or descriptive notes on all previously described species, describe Mesovelia ebbenielseni sp. nov. (Mesoveliidae), Austrohebrus apterus, gen. et sp. nov., and Hebrus pilosus sp. nov. (Hebridae), and synonymise Hebrus woodwardi Lansbury, syn. nov. (Hebridae) and Hydrometra halei Hungerford and Evans, syn. nov. (Hydrometridae). We present keys for the identification of genera and species, and map the distribution of all species. -

Appendix 5: Summary of Existing Strategies, Policies and Plans
Appendiix 5: Summary of exiistiing strategiies, polliiciies and plans Within each section of this appendix, the various international and national strategies and plans are presented first, followed by State, regional and locally relevant documents. The major sections are as follows: 1 Biodiversity ...................................................................................................................................... 1 2 Migratory species............................................................................................................................. 7 3 Natural resouce management targets ............................................................................................. 8 4 Pests and weeds ........................................................................................................................... 10 5 Strategic landuse planning ............................................................................................................ 15 6 Threatened species ....................................................................................................................... 17 7 Water, wetlands and estuaries ...................................................................................................... 20 8 Coastal ........................................................................................................................................... 29 1 Biiodiversiity International Convention on Biological Diversity 1992 Level: International Relevance: Contributes Author: -

Northern Rivers Regional Biodiversity Management Plan
Foreword The Northern Rivers Regional Biodiversity Management Plan (the Plan) constitutes the national regional recovery plan under the Environment Protection and Biodiversity Conservation Act 1999 for threatened species and ecological communities principally distributed in the Northern Rivers Region of NSW. The Plan is part of an Australian Government-funded pilot to trial the integration of regional recovery and threat abatement planning. It provides a regional approach to the delivery of recovery actions necessary to ensure the long-term viability of threatened species and ecological communities in the Region. The Northern Rivers Region is an area relatively rich in biodiversity data. This has allowed for innovative and sophisticated analysis techniques to be used in this Plan for biodiversity assessment and identification of priority areas for conservation works. These outputs will help guide investment by the Australian Government, New South Wales Government and local authorities in the Region. Collaboration and partnerships will be essential for the implementation of the Plan. The Plan considers all threats affecting biodiversity in the Region, including those associated with the potential impacts of climate change. The Plan also incorporates Indigenous cultural values and considerations into biodiversity management in the Region. It is in this context that the Plan, in association with the approved Border Ranges Rainforest Biodiversity Management Plan (DECCW 2010), sets out an overall strategy for the conservation and restoration