The History and Impact of the CNO Cycles in Nuclear Astrophysics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Simulating Physics with Computers
International Journal of Theoretical Physics, VoL 21, Nos. 6/7, 1982 Simulating Physics with Computers Richard P. Feynman Department of Physics, California Institute of Technology, Pasadena, California 91107 Received May 7, 1981 1. INTRODUCTION On the program it says this is a keynote speech--and I don't know what a keynote speech is. I do not intend in any way to suggest what should be in this meeting as a keynote of the subjects or anything like that. I have my own things to say and to talk about and there's no implication that anybody needs to talk about the same thing or anything like it. So what I want to talk about is what Mike Dertouzos suggested that nobody would talk about. I want to talk about the problem of simulating physics with computers and I mean that in a specific way which I am going to explain. The reason for doing this is something that I learned about from Ed Fredkin, and my entire interest in the subject has been inspired by him. It has to do with learning something about the possibilities of computers, and also something about possibilities in physics. If we suppose that we know all the physical laws perfectly, of course we don't have to pay any attention to computers. It's interesting anyway to entertain oneself with the idea that we've got something to learn about physical laws; and if I take a relaxed view here (after all I'm here and not at home) I'll admit that we don't understand everything. -

PP and CNO-Cycle Nucleosynthesis: Kinetics and Numerical Modeling of Competitive Fusion Processes
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Supervised Undergraduate Student Research Chancellor’s Honors Program Projects and Creative Work 5-2012 PP and CNO-Cycle Nucleosynthesis: Kinetics and Numerical Modeling of Competitive Fusion Processes Matt Torrico [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_chanhonoproj Part of the Physical Processes Commons, and the Plasma and Beam Physics Commons Recommended Citation Torrico, Matt, "PP and CNO-Cycle Nucleosynthesis: Kinetics and Numerical Modeling of Competitive Fusion Processes" (2012). Chancellor’s Honors Program Projects. https://trace.tennessee.edu/utk_chanhonoproj/1557 This Dissertation/Thesis is brought to you for free and open access by the Supervised Undergraduate Student Research and Creative Work at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Chancellor’s Honors Program Projects by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. PP and CNO-Cycle Nucleosynthesis: Kinetics and Numerical Modeling of Competitive Fusion Processes M.N. TorricoA, M.W. GuidryA,B A Department of Physics and Astronomy, University of Tennessee, Knoxville, TN 37996, USA B Physics Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA Signed on 21 April 2012 Abstract The very history of matter (and hence Man) is exquisitely coupled to the nuclear fusion processes that power the Sun and other stars. The fusion of hydrogen into helium and other thermonuclear fusion processes (collectively called nucleosynthesis processes) provides us with not only the energy to carry on our lives, but the very materials that constitute our very bodies and our world. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

Cockcroft: a Novel This One, the Comprehensive Compilation Lutely Fundamental Discovery
~7~~-------------------------------------SPRINGBCXJKS------------------------N_A_ru_~__ v_o_L._Jo_s_~_AP __ Rr_L_r9M_ same, when it's compiled as successfully as what was in the sheer physics of it an abso Cockcroft: a novel this one, the comprehensive compilation lutely fundamental discovery. The authors perspective undeniably earns its place in the library. of this book take in their stride Cockcroft I was an admirer of Cockcroft, a fine, and Walton's being made to wait so long William Cooper stocky, sensible, cautious Yorkshireman, theirs not to reason why, they say. But what who indulged in a minimum of speech and about ours? Did it take the Nobel Cockcroft and the Atom. in practically no change of facial committee nineteen years to recognize the By Guy Hartcup and T.E. Allibone. expression. (That expression was neverthe experiment as breaking open an entirely Adam Hilger, Bristol!Heyden, Phila- less amiable, thoughtful, smiling.) If one new line of powerful experiments to be delphia: 1984. Pp.320. £18.95, $34. addressed a remark to him, it was only made with particle-accelerators? Or did it when, after the ensuing silence, he either take those members who opposed the took the famous black notebook out of his award all that time to disappear from the SrNCE the title of Hartcup and Allibone's pocket or actually said something, that one scene? Or what? Someone must know. book may sound a bit vague, perhaps I knew for certain that he'd heard. It gave me There's a fascinating detail I should really should begin by saying what Cockcroft and lasting pleasure to think that at a dinner like to be told. -

Alfred O. C. Nier
CHEMICAL HERITAGE FOUNDATION ALFRED O. C. NIER Transcript of Interviews Conducted by Michael A. Grayson and Thomas Krick at University of Minnesota Minneapolis, Minnesota on 7, 8, 9, and 10 April 1989 (With Subsequent Corrections and Additions) ACKNOWLEDGMENT This oral history is one in a series initiated by the Chemical Heritage Foundation on behalf of the American Society for Mass Spectrometry. The series documents the personal perspectives of individuals related to the advancement of mass spectrometric instrumentation, and records the human dimensions of the growth of mass spectrometry in academic, industrial, and governmental laboratories during the twentieth century. This project is made possible through the generous support of the American Society for Mass Spectrometry Upon Alfred O.C. Nier’s death in 1994, this oral history was designated Free Access. Please note: Users citing this interview for purposes of publication are obliged under the terms of the Chemical Heritage Foundation Oral History Program to credit CHF using the format below: Alfred O.C. Nier, interview by Michael A. Grayson and Thomas Krick at the University of Minnesota, Minneapolis, Minnesota, 7-10 April 1989 (Philadelphia: Chemical Heritage Foundation, Oral History Transcript # 0112). Chemical Heritage Foundation Oral History Program 315 Chestnut Street Philadelphia, Pennsylvania 19106 The Chemical Heritage Foundation (CHF) serves the community of the chemical and molecular sciences, and the wider public, by treasuring the past, educating the present, and inspiring the future. CHF maintains a world-class collection of materials that document the history and heritage of the chemical and molecular sciences, technologies, and industries; encourages research in CHF collections; and carries out a program of outreach and interpretation in order to advance an understanding of the role of the chemical and molecular sciences, technologies, and industries in shaping society. -

Bibliography
Bibliography F.H. Attix, Introduction to Radiological Physics and Radiation Dosimetry (John Wiley & Sons, New York, New York, USA, 1986) V. Balashov, Interaction of Particles and Radiation with Matter (Springer, Berlin, Heidelberg, New York, 1997) British Journal of Radiology, Suppl. 25: Central Axis Depth Dose Data for Use in Radiotherapy (British Institute of Radiology, London, UK, 1996) J.R. Cameron, J.G. Skofronick, R.M. Grant, The Physics of the Body, 2nd edn. (Medical Physics Publishing, Madison, WI, 1999) S.R. Cherry, J.A. Sorenson, M.E. Phelps, Physics in Nuclear Medicine, 3rd edn. (Saunders, Philadelphia, PA, USA, 2003) W.H. Cropper, Great Physicists: The Life and Times of Leading Physicists from Galileo to Hawking (Oxford University Press, Oxford, UK, 2001) R. Eisberg, R. Resnick, Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles (John Wiley & Sons, New York, NY, USA, 1985) R.D. Evans, TheAtomicNucleus(Krieger, Malabar, FL USA, 1955) H. Goldstein, C.P. Poole, J.L. Safco, Classical Mechanics, 3rd edn. (Addison Wesley, Boston, MA, USA, 2001) D. Greene, P.C. Williams, Linear Accelerators for Radiation Therapy, 2nd Edition (Institute of Physics Publishing, Bristol, UK, 1997) J. Hale, The Fundamentals of Radiological Science (Thomas Springfield, IL, USA, 1974) W. Heitler, The Quantum Theory of Radiation, 3rd edn. (Dover Publications, New York, 1984) W. Hendee, G.S. Ibbott, Radiation Therapy Physics (Mosby, St. Louis, MO, USA, 1996) W.R. Hendee, E.R. Ritenour, Medical Imaging Physics, 4 edn. (John Wiley & Sons, New York, NY, USA, 2002) International Commission on Radiation Units and Measurements (ICRU), Electron Beams with Energies Between 1 and 50 MeV,ICRUReport35(ICRU,Bethesda, MD, USA, 1984) International Commission on Radiation Units and Measurements (ICRU), Stopping Powers for Electrons and Positrons, ICRU Report 37 (ICRU, Bethesda, MD, USA, 1984) 646 Bibliography J.D. -

Merle A. Tuve
NATIONAL ACADEMY OF SCIENCES M E R L E A N T O N Y T UVE 1901—1982 A Biographical Memoir by P H ILI P H . AbELSON Any opinions expressed in this memoir are those of the author(s) and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoir COPYRIGHT 1996 NATIONAL ACADEMIES PRESS WASHINGTON D.C. MERLE ANTONY TUVE June 27, 1901–May 20, 1982 BY PHILIP H. ABELSON ERLE ANTONY TUVE WAS a leading scientist of his times. MHe joined with Gregory Breit in the first use of pulsed radio waves in the measurement of layers in the ionosphere. Together with Lawrence R. Hafstad and Norman P. Heydenburg he made the first and definitive measurements of the proton-proton force at nuclear distances. During World War II he led in the development of the proximity fuze that stopped the buzz bomb attack on London, played a crucial part in the Battle of the Bulge, and enabled naval ships to ward off Japanese aircraft in the western Pacific. Following World War II he served for twenty years as director of the Carnegie Institution of Washington’s Department of Ter- restrial Magnetism, where, in addition to supporting a mul- tifaceted program of research, he personally made impor- tant contributions to experimental seismology, radio astronomy, and optical astronomy. Tuve was a dreamer and an achiever, but he was more than that. He was a man of conscience and ideals. Throughout his life he remained a scientist whose primary motivation was the search for knowledge but a person whose zeal was tempered by a regard for the aspirations of other humans. -
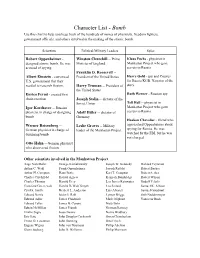
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Lecture 7: "Basics of Star Formation and Stellar Nucleosynthesis" Outline
Lecture 7: "Basics of Star Formation and Stellar Nucleosynthesis" Outline 1. Formation of elements in stars 2. Injection of new elements into ISM 3. Phases of star-formation 4. Evolution of stars Mark Whittle University of Virginia Life Cycle of Matter in Milky Way Molecular clouds New clouds with gravitationally collapse heavier composition to form stellar clusters of stars are formed Molecular cloud Stars synthesize Most massive stars evolve He, C, Si, Fe via quickly and die as supernovae – nucleosynthesis heavier elements are injected in space Solar abundances • Observation of atomic absorption lines in the solar spectrum • For some (heavy) elements meteoritic data are used Solar abundance pattern: • Regularities reflect nuclear properties • Several different processes • Mixture of material from many, many stars 5 SolarNucleosynthesis abundances: key facts • Solar• Decreaseabundance in abundance pattern: with atomic number: - Large negative anomaly at Be, B, Li • Regularities reflect nuclear properties - Moderate positive anomaly around Fe • Several different processes 6 - Sawtooth pattern from odd-even effect • Mixture of material from many, many stars Origin of elements • The Big Bang: H, D, 3,4He, Li • All other nuclei were synthesized in stars • Stellar nucleosynthesis ⇔ 3 key processes: - Nuclear fusion: PP cycles, CNO bi-cycle, He burning, C burning, O burning, Si burning ⇒ till 40Ca - Photodisintegration rearrangement: Intense gamma-ray radiation drives nuclear rearrangement ⇒ 56Fe - Most nuclei heavier than 56Fe are due to neutron -

{Download PDF} Feynman
FEYNMAN PDF, EPUB, EBOOK Jim Ottaviani | 272 pages | 13 May 2013 | Roaring Brook Press | 9781596438279 | English | New Milford, United States The Feynman Technique: A Beginner's Guide to Learning Fast While complex, subject-specific jargon sounds cool, it confuses people and urges them to stop paying attention. Replace technical terms with simpler words, and think of how you could explain your lesson to a child. Children are not able to understand jargon or dense vocabulary. His charts were able to simply explain things that other scientists took hours to lecture students on in an attempt to teach them. If a concept is highly technical or complicated, analogies are also a good way to simplify them. Analogies are the foundation of learning from experience, and they work because they make use of your brain's natural inclination to match patterns. Analogies influence what you perceive and remember, and help you process information more easily because you associate it with things you already know. These mental shortcuts are useful methods of processing new and unfamiliar information and help people understand, organize, and comprehend incoming information. One example of an analogy created by Feynman encapsulates the power of his technique. He was able to take a question regarding human existence and simplify it into a simple sentence that even a middle-schooler could understand. Feynman said:. Here, Feynman is saying that if you don't know anything about physics, the most important concept to understand is that everything is composed of atoms. In one sentence, he communicates the fundamental existence of the universe. -

Pp-Chain and CNO Cycle
10 – pp-chain and CNO cycle introduc)on to Astrophysics, C. Bertulani, Texas A&M-Commerce 1 pp chain reaction in the Sun introduc)on to Astrophysics, C. Bertulani, Texas A&M-Commerce 2 10.1 - Hydrogen burning – ppI chain As a star forms from a giant cloud in the interstellar medium (protostar), density and temperature increase in its center. Fusion of hydrogen (1H) is the first long term nuclear energy source that can ignite. With only hydrogen available (for example, a first generation star) the ppI chain (see previous slide) is the only possible sequence of reactions. (other reaction chains require catalyst nuclei). The ppI chain proceeds as follows: 2 Step 1: p + p → He This does not work because 2He is unstable + (10.1) p + p → d + e + ve 3 (10.2) Step 2: d + p → He d d 4 He This does not work because the d abundance is + → too low. d + p leads to rapid destruction of d. 3 4 Step 3: He + p → Li Does not work because 4Li is unstable 3 He + d → 4 He + n Does not work because d abundance is too low 3 He + 3 He → 4 He + 2p (10.3) Last reaction is OK because Y3He gets large as there is no other rapid destruction. introduc)on to Astrophysics, C. Bertulani, Texas A&M-Commerce 3 10.1.1 - S-factors for the pp chain All reactions in the pp-chain are charge-dependent. The cross section has the form given by Eq. (9.49) which can be cast in the form b − where 1 E σ ∝ e (10.4) A1A2 E b 31.28 Z Z A1/2 keV A = = 1 2 (10.5) A1 + A2 (10.6) Typical units for S(E) are keV barn. -

Trinity Transcript
THE NATIONAL ACADEMIES Committee on International Security and Arms Control 60th Anniversary of Trinity: First Manmade Nuclear Explosion, July 16, 1945 PUBLIC SYMPOSIUM July 14, 2005 National Academy of Sciences Auditorium 2100 C Street, NW Washington, DC Proceedings By: CASET Associates, Ltd. 10201 Lee Highway, Suite 180 Fairfax, VA 22030 (703) 352-0091 CONTENTS PAGE Introductory Remarks Welcome: Ralph Cicerone, President, The National Academies (NAS) 1 Introduction: Raymond Jeanloz, Chair, Committee on International Security and Arms Control (CISAC) 3 Roundtable Discussion by Trinity Veterans Introduction: Wolfgang Panofsky, Chair 5 Individual Statements by Trinity Veterans: Harold Agnew 10 Hugh Bradner 13 Robert Christy 16 Val Fitch 20 Don Hornig 24 Lawrence Johnston 29 Arnold Kramish 31 Louis Rosen 35 Maurice Shapiro 38 Rubby Sherr 41 Harold Agnew (continued) 43 1 PROCEEDINGS 8:45 AM DR. JEANLOZ: My name is Raymond Jeanloz, and I am the Chair of the Committee on International Security and Arms Control that organized this morning’s symposium, recognizing the 60th anniversary of Trinity, the first manmade nuclear explosion. I will be the moderator for today’s event, and primarily will try to stay out of the way because we have many truly distinguished and notable speakers. In order to allow them the maximum amount of time, I will only give brief introductions and ask that you please turn to the biographical information that has been provided to you. To start with, it is my special honor to introduce Ralph Cicerone, the President of the National Academy of Sciences, who will open our meeting with introductory remarks. He is a distinguished researcher and scientific leader, recently serving as Chancellor of the University of California at Irvine, and his work in the area of climate change and pollution has had an important impact on policy.