Life History and Population Dynamics of Lake Sturgeon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pallid Sturgeon Recovery Plan (Recovery Plan)
PALLID STURGEON coRE VERYPMN Recovery Plan for the Pallid Sturgeon (Scaphirhynchus a/bus) Prepared by the Pallid Sturgeon Recovery Team Principal Authors Mark P. Dryer, Leader U.S. Fish and Wildlife Service Ecological Services 1500 Capitol Avenue Bismarck, ND 58501 and Alan J. Sandvol U.S. Fish and Wildlife Service Fisheries and Federal Aid 1500 Capitol Avenue Bismarck, ND 58501 for Region 6 U.S. Fish and Wildlife Service Denver, Colorado / Approved: Re~5l i rector Date TABLE OF CONTENTS TITLE PAGE RECOVERY BACKGROUND AND STRATEGY iii PALLID STURGEON RECOVERY TEAM iv DISCLAIMER V ACKNOWLEDGEMENTS vi EXECUTIVE SUMMARY vii Part I INTRODUCTION 1 History 1 General Description 1 Historical Distribution and Abundance 3 Present Distribution and Abundance 5 Habitat Preference 5 Current Velocity 7 Turbidity 8 Water Depth 8 Substrate 8 Temperature 8 Life History 8 Reproductive Biology 8 Food and Feeding Habits 9 Age and Growth 10 Reasons for Decline 10 Habitat Loss 10 Commercial Harvest 13 Pall uti on/Contami nants 14 Hybridization 14 Part II RECOVERY 16 Recovery Objectives and Criteria 16 Recovery—Priority Management Areas 16 Recovery Outline 19 24 Recovery Outline Narrative . LITERATURE CITED 42 Part III IMPLEMENTATION SCHEDULE 46 FIGURES NO. PAGE 1. Comparative Diagrams of the Ventral Surface of the Head of Shovelnose Sturgeon and Pallid Sturgeon, Showing Several Measurement Ratios of Value for Identification 2 2. Historic Range of Pallid Sturgeon 4 3. Recent Occurrence of Pallid Sturgeon 6 4. Recovery—Priority Management Areas 18 RECOVERY BACKGROUND AND STRATEGY The pallid sturgeon (Scaphirhynchus albus Forbes and Richardson) was listed as an endangered species on September 6, 1990 (55 FR 36641) pursuant to the Endangered Species Act (Act) of 1973 (16 U.S.C. -

Status of Lake Sturgeon (Acipenser Fulvescens Rafinesque 1817)
Journal of Applied Ichthyology J. Appl. Ichthyol. 32 (Suppl. 1) (2016), 162–190 Received: August 12, 2016 © 2016 Blackwell Verlag GmbH Accepted: October 19, 2016 ISSN 0175–8659 doi: 10.1111/jai.13240 Status of Lake Sturgeon (Acipenser fulvescens Rafinesque 1817) in North America By R. M. Bruch1, T. J. Haxton2, R. Koenigs1, A. Welsh3 and S. J. Kerr2 1Wisconsin Department of Natural Resources, Oshkosh, WI, USA; 2Ontario Ministry of Natural Resources and Forestry, Peterborough, ON, Canada; 3School of Natural Resources, West Virginia University, Morgantown, WV, USA Summary The species Lake Sturgeon (LS) had been assigned at least Lake Sturgeon is a potamodromous, fluvial-dependent spe- 17 different scientific names during the 19th and 20th cen- cies from the family Acipenseridae, and one of the largest turies due to the variation in color and shape displayed by freshwater fishes within its North American range extending the different life stages and populations (Scott and Cross- to the Great lakes, Mississippi River, and Hudson Bay drai- man, 1973). Eventually LS was recognized as one species nages. Like almost all other sturgeon species, Lake Stur- with the scientific designation Acipenser fulvescens (ful- = geon populations throughout its range suffered mass vescens yellowish or tawny) originally proposed by Con- – declines or extirpation in the late 1800s into the early stantine Samuel Rafinesque (1783 1840). The common name 1900s, due to extensive overexploitation and habitat loss Lake Sturgeon was given due to the abundance of the species and -

Summary of Trout and Salmon Stocking in Lake Michigan 1976-2002
Lake Michigan Committee Meeting Milwaukee, Wisconsin March 19-20, 2003 Summary of Trout and Salmon Stocking in Lake Michigan 1976-2002 Prepared by Jessica M. Richards and Charles R. Bronte U.S. Fish & Wildlife Service Fishery Resources Office 2661 Scott Tower Drive New Franken, WI 54229 This report summarizes trout and salmon stocking in Lake Michigan from 1976-2002. Stocking information was provided by the Department of Natural Resources of Illinois, Indiana, Michigan and Wisconsin, who stock Pacific salmon, brown trout, and splake, and the U.S. Fish and Wildlife Service, who provides all lake trout for restoration purposes. These data should be considered provisional because not all agencies have officially verified the stocking numbers. With the exception of lake trout, no attempt was made to express stocking totals in yearling equivalents. Consequently, numbers for all life stages stocked for a given species were added together. To accurately examine trends in adult trout and salmon abundance in Lake Michigan, detailed information on hatchery programs, genetic strains, size at stocking, and stocking practices would have to be considered. Lakewide Trends: The number of trout and salmon stocked into Lake Michigan in 2002 totaled 12.0 million fish (Table 1), which was about 1.0 million fewer fish than last year. This level of stocking was 2.4 million less than the long term average of 14.5 million, and one of the lowest levels since 1977. Total stocking peaked at 17.3 million fish in 1984. Chinook Salmon: Chinook salmon stocking in Lake Michigan decreased to 3.3 million fish in 2002, which was about 1.0 million fewer fish than last year (Table 1). -

2012 Wildearth Guardians and Friends of Animals Petition to List
PETITION TO LIST Fifteen Species of Sturgeon UNDER THE U.S. ENDANGERED SPECIES ACT Submitted to the U.S. Secretary of Commerce, Acting through the National Oceanic and Atmospheric Administration and the National Marine Fisheries Service March 8, 2012 Petitioners WildEarth Guardians Friends of Animals 1536 Wynkoop Street, Suite 301 777 Post Road, Suite 205 Denver, Colorado 80202 Darien, Connecticut 06820 303.573.4898 203.656.1522 INTRODUCTION WildEarth Guardians and Friends of Animals hereby petitions the Secretary of Commerce, acting through the National Marine Fisheries Service (NMFS)1 and the National Oceanic and Atmospheric Administration (NOAA) (hereinafter referred as the Secretary), to list fifteen critically endangered sturgeon species as “threatened” or “endangered” under the Endangered Species Act (ESA) (16 U.S.C. § 1531 et seq.). The fifteen petitioned sturgeon species, grouped by geographic region, are: I. Western Europe (1) Acipenser naccarii (Adriatic Sturgeon) (2) Acipenser sturio (Atlantic Sturgeon/Baltic Sturgeon/Common Sturgeon) II. Caspian Sea/Black Sea/Sea of Azov (3) Acipenser gueldenstaedtii (Russian Sturgeon) (4) Acipenser nudiventris (Ship Sturgeon/Bastard Sturgeon/Fringebarbel Sturgeon/Spiny Sturgeon/Thorn Sturgeon) (5) Acipenser persicus (Persian Sturgeon) (6) Acipenser stellatus (Stellate Sturgeon/Star Sturgeon) III. Aral Sea and Tributaries (endemics) (7) Pseudoscaphirhynchus fedtschenkoi (Syr-darya Shovelnose Sturgeon/Syr Darya Sturgeon) (8) Pseudoscaphirhynchus hermanni (Dwarf Sturgeon/Little Amu-Darya Shovelnose/Little Shovelnose Sturgeon/Small Amu-dar Shovelnose Sturgeon) (9) Pseudoscaphirhynchus kaufmanni (False Shovelnose Sturgeon/Amu Darya Shovelnose Sturgeon/Amu Darya Sturgeon/Big Amu Darya Shovelnose/Large Amu-dar Shovelnose Sturgeon/Shovelfish) IV. Amur River Basin/Sea of Japan/Sea of Okhotsk (10) Acipenser mikadoi (Sakhalin Sturgeon) (11) Acipenser schrenckii (Amur Sturgeon) (12) Huso dauricus (Kaluga) V. -
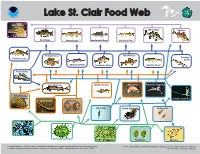
Lake St. Clair Food Web MENT of C
ATMOSPH ND ER A I C C I A N D A M E I C N O I S L T A R N A T O I I O T N A N U E .S C .D R E E PA M RT OM Lake St. Clair Food Web MENT OF C Sea Lamprey White Bass Walleye Rock Bass Muskellunge Smallmouth Bass Northern Pike Pumpkinseed Yellow Perch Rainbow Common Carp Smelt Channel Catfish Freshwater Drum Spottail Shiner Round Goby Lake Sturgeon Mayfly nymphs Gammarus Raptorial waterflea Zebra/Quagga mussels Invasive waterflea Chironomids Mollusks Calanoids Native waterflea Cyclopoids Diatoms Green algae Blue-green algae Flagellates Rotifers Foodweb based on “Impact of exotic invertebrate invaders on food web structure and function in the Great Lakes: NOAA, Great Lakes Environmental Research Laboratory, 4840 S. State Road, Ann Arbor, MI A network analysis approach” by Mason, Krause, and Ulanowicz, 2002 - Modifications for Lake St. Clair, 2009. 734-741-2235 - www.glerl.noaa.gov Lake St. Clair Food Web Sea Lamprey Planktivores/Benthivores Sea lamprey (Petromyzon marinus). An aggressive, non-native parasite that Lake Sturgeon (Acipenser fulvscens). Endangered over most of its historic fastens onto its prey and rasps out a hole with its rough tongue. range. Its diet commonly includes small clams, snails, crayfish, sideswimmers, aquatic insect larvae, algae, and other plant matter. Piscivores (Fish Eaters) Macroinvertebrates White bass (Morone Chrysops). Prefers clear open water in lakes and large rivers. Chironomids/Oligochaetes. Larval insects and worms that live on the lake Visual feeders, uses sight instead of smell to find prey. -

Take Me to the River
July 2011 Free © northerncamper.com Pine, Muskegon, & Manistee Take Me to the River Recipes Festivities Puzzles American Legion Post 300 Open to public July 1-2 With Ron on Oneal July 1 Small TOWN 8 -11 p.m. Marshue’s Tantastic Boutique Tanning, Body Wraps, Gifts Bait Shop North 231-839-TANS 231-839-FISH “Where you are brown year ‘round” Stops Full Line of Bait & Tackle Beach Supplies, Pop, Snacks The Coffee Cup See Ad Below Baldwin Wood Products 231-839-0042 The Missaukee Sentinel Special Orders Taken Missaukee County News Source Open 7 Days a Week, 7 - ? Copy/Fax, Office Supplies, Dry Cleaning 231-839-5400, missaukeesentinel.com Cadillac Mortgage 41 N. Morey Rd. The Town Pump 231-839-0600 See Ad Below www.cadillacmortgage.com Videos North Curves of Lake City Mon. - Thurs., Noon - 9 p.m. 57 N. Morey Rd. (Across from McDonald’s) Fri. - Sun., Noon - 10 p.m. 231-839-6889 105 South Main Street 30 Minute Workout, Fun/Fast/Safe WhiteTail Realty In The North! The In Don’s American Pizza See Ad Page 3 th Open July 4th 231-839-2670 Woodstock Gifts Homemade Pizza, Pepperoni Rolls Up North Lodge Decor Unique & Practical Gifts Larsen’s Early Learning Center Lots of Tee Shirts & Sweatshirts Ages 2½ - 12, D.H.S. Accepted Building Block to Your Child’s Future 231-839-7779 Have Fun In Lake City Lake In Fun Have Greatest 4 Greatest Town Pump The Coffee Cup Biggest Breakfast In the North! Editor’s Editor’s choice best breakfast! choice coldest Saloon 7 am – 3 pm • 7 Days • Daily Specials beer! Keno • Pull Tabs • Pool Locally Owned & Operated 5 Large Screen TVs • 42" Plasma TV 231-839-4859 Burgers & Pizza • Coney Islands (Saturdays) 84 N. -

Yangtze Sturgeon (Acipenser Dabryanus) - Sturgeons
Pond Life - Yangtze Sturgeon (Acipenser dabryanus) - Sturgeons http://www.pond-life.me.uk/sturgeon/acipenserdabryanus.php Search Pond Life... Home Sturgeons Koi Other Fish Fish Health Ponds Plants Forums Contents Yangtze Sturgeon (Acipenser dabryanus) Home Sturgeons Acipenseriformes Sturgeon Food & Feeding Sturgeon Care Sheet Sturgeon Guide Sturgeon Species List Adriatic Sturgeon Alabama Sturgeon Amu Darya Sturgeon Amur Sturgeon Atlantic Sturgeon Beluga Sturgeon Chinese Paddlefish Chinese Sturgeon Yangtze Sturgeon (Acipenser dabryanus) photo from the website of CAFS Common Sturgeon (http://zzzy.fishinfo.cn/) Diamond Sturgeon Dwarf Sturgeon by Karen Paul Green Sturgeon Description: The Yangtze Sturgeon (Acipenser dabryanus) has 8-13 dorsal scutes, 26-39 lateral Gulf Sturgeon scutes, 9-13 ventral scutes, 44-57 dorsal fin rays and 25-36 anal fin rays. Colouration ranges from Kaluga Sturgeon dark grey to brown-grey on the back to white on the ventral side. The body is rough because it is Lake Sturgeon covered with small pointed denticles. The four barbels are located closer to the mouth than the end Paddlefish of the snout. The Yangtze Sturgeon can reach 1.3 meters in length and a weight of 16kg. Pallid Sturgeon Persian Sturgeon Sakhalin Sturgeon Ship Sturgeon Shortnose Sturgeon Shovelnose Sturgeon Siberian Sturgeon Stellate Sturgeon Sterlet Syr Darya Sturgeon White Sturgeon Yangtze Sturgeon Sturgeon Videos Koi Other Fish Fish Health Yangtze Sturgeon (Acipenser dabryanus) photo from the website of CAFS Ponds (http://zzzy.fishinfo.cn/) Plants Forums Wild Distribution: Asia; restricted to the upper and middle reaches of the Yangtze River system, Search rarely seen below the Gezhouba Dam. The Yangtze Sturgeon is a potamodromous (freshwater only) species. -

Petition to List US Populations of Lake Sturgeon (Acipenser Fulvescens)
Petition to List U.S. Populations of Lake Sturgeon (Acipenser fulvescens) as Endangered or Threatened under the Endangered Species Act May 14, 2018 NOTICE OF PETITION Submitted to U.S. Fish and Wildlife Service on May 14, 2018: Gary Frazer, USFWS Assistant Director, [email protected] Charles Traxler, Assistant Regional Director, Region 3, [email protected] Georgia Parham, Endangered Species, Region 3, [email protected] Mike Oetker, Deputy Regional Director, Region 4, [email protected] Allan Brown, Assistant Regional Director, Region 4, [email protected] Wendi Weber, Regional Director, Region 5, [email protected] Deborah Rocque, Deputy Regional Director, Region 5, [email protected] Noreen Walsh, Regional Director, Region 6, [email protected] Matt Hogan, Deputy Regional Director, Region 6, [email protected] Petitioner Center for Biological Diversity formally requests that the U.S. Fish and Wildlife Service (“USFWS”) list the lake sturgeon (Acipenser fulvescens) in the United States as a threatened species under the federal Endangered Species Act (“ESA”), 16 U.S.C. §§1531-1544. Alternatively, the Center requests that the USFWS define and list distinct population segments of lake sturgeon in the U.S. as threatened or endangered. Lake sturgeon populations in Minnesota, Lake Superior, Missouri River, Ohio River, Arkansas-White River and lower Mississippi River may warrant endangered status. Lake sturgeon populations in Lake Michigan and the upper Mississippi River basin may warrant threatened status. Lake sturgeon in the central and eastern Great Lakes (Lake Huron, Lake Erie, Lake Ontario and the St. Lawrence River basin) seem to be part of a larger population that is more widespread. -

Round Goby (Neogobius Melanostomus), Native to the Black Sea, Threatens the Waters of the State of Michigan
State of Michigan’s Status and Strategy for Round Goby Management Scope The invasion of round goby (Neogobius melanostomus), native to the Black Sea, threatens the waters of the State of Michigan. The goals of this document are to summarize the: • Current level of understanding on the biology and ecology of the round goby. • Management options for round goby in Michigan. • Future possible directions for round goby management in Michigan. Biology and Ecology I. Identification Round gobies are small, soft- bodied fish. Their fused pelvic fin forms a suction disk on their ventral surface and distinguishes them from native sculpins. Adults have brownish gray bodies with dark brown/black blotches. During spawning and nest guarding, males have black bodies with yellowish spots and median fins with a yellow/white outer edge. Generally, a large, oblong, black spot is present at the end of the first white/greenish dorsal fin, beginning at the fifth ray; sculpins usually have a mark in the same area. This black spot on juveniles has a light border. The round goby’s head that makes up approximately 22 to 23% of its body length is as wide or wider than it is deep (Charlesbois et al. 1997). In the United States, round gobies reach up to 17.8 cm in length (Jude 1993). II. Life History In the spring, when water temperatures range from 9 to 26°C, males migrate from deeper water before the females to establish spawning areas in shallow water (Moiseyeva and Rudenko 1976, MacInnis and Corkum 2000, Murphy et al. 2001, Kornis et al. -

Caviar and Conservation
Caviar and Conservation Status, Management, and Trade of North American Sturgeon and Paddlefish Douglas F.Williamson May 2003 TRAFFIC North America World Wildlife Fund 1250 24th Street NW Washington DC 20037 Visit www.traffic.org for an electronic edition of this report, and for more information about TRAFFIC North America. © 2003 WWF. All rights reserved by World Wildlife Fund, Inc. All material appearing in this publication is copyrighted and may be reproduced with permission. Any reproduction, in full or in part, of this publication must credit TRAFFIC North America. The views of the author expressed in this publication do not necessarily reflect those of the TRAFFIC Network, World Wildlife Fund (WWF), or IUCN-The World Conservation Union. The designation of geographical entities in this publication and the presentation of the material do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organizations concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The TRAFFIC symbol copyright and Registered Trademark ownership are held by WWF. TRAFFIC is a joint program of WWF and IUCN. Suggested citation: Williamson, D. F. 2003. Caviar and Conservation: Status, Management and Trade of North American Sturgeon and Paddlefish. TRAFFIC North America. Washington D.C.: World Wildlife Fund. Front cover photograph of a lake sturgeon (Acipenser fulvescens) by Richard T. Bryant, courtesy of the Tennessee Aquarium. Back cover photograph of a paddlefish (Polyodon spathula) by Richard T. Bryant, courtesy of the Tennessee Aquarium. TABLE OF CONTENTS Preface . -

Sturgeons – Contemporaries of Dinosaurs
G.M. PALATNIKOV, R.U. KASIMOV STURGEONS – CONTEMPORARIES OF DINOSAURS BAKU – 2010. INTRODUCTION TheCaspian Sea is abundant with its natural unique gems, the most famous being sturgeons and oil. But, as the old wisdom says, wealth alone will make no one happy, especially if used without thinking of future. All natural resources can be categorized as renewables and nonrenewables. Renewables are such resources that can regenerate through reproduction or through the nature renewal cycles over the time frame comparable with the pace of huuman economical activity. Examples of such resources are sturgeons. Nonrenewables are such resources that cannot regenerate over the natural cycle over the time frame comparable with the pace of human economical activity. Oil reserves are such nonrenewable resources. Oil has now been actively developed and produced. Big money is being spent for the development of hydrocarbon exploration, production and transportation technologies. But hydrocarbon reserves sooner or later will deplete. As for sturgeons, their stock is being gradually decreased because of irrational catch, reduction of feeding and spawning areas, and contamination of the water environment - and oil industry has contributed greatly to it. However, this problem has currently not been addressed properly although preservation of sturgeon reserves requires less investment but promises continuously high profits at all times. Unfortunately, not everyone, including those who live at the Caspian Sea, understands this. Most people have insufficient knowledge about sturgeons, but view sturgeons as a source of food. We will try to inform the readers about the life of this unique fish. We will tell you the following: What are these species that have left such a noticeable footprint in the history of mankind? What y interest (other than being a source of food) do they present? Will they be preserved for future generations, or disappear as did their contemporaries – the dinosaurs? 1 GOING BACK TO HISTORY Sturgeon catching has been known since ancient times. -

Get Closer to Nature Enjoy Michigan’S Rivers and Natural Woodlands
GET CLOSER TO NATURE ENJOY MICHIGAN’S RIVERS AND NATURAL WOODLANDS. A LITTLE WILD, A LOT WONDERFUL CONSUMERS ENERGY GET CLOSER TO NATURE • 1 GET CLOSER TO NATURE A LITTLE WILD, A LOT WONDERFUL xperience the best nature has to offer. Whether it’s a leisurely bike ride or hike through miles Eof pine forests with hardly another human passerby. Built and operated by Consumers Rogers Hydro Energy since the early 1900s, the 12,000 acres of land and water at our 13 hydro- electric dams offer many recreational opportunities: Au Sable River Mio • Fishing or camping. Alcona • Picnicking and swimming. Manistee River Cooke • Canoeing and seeing a deer take a Hodenpyl PAGE Foote PAGE drink at the edge of the water. Five Channels • Catching sight of a bald eagle soaring Tippy 6 Loud high above its nest or a family of 4 trumpeter swans gliding silently across Muskegon River the water. Hardy • Viewing Michigan’s fall foilage along Rogers PAGE the brilliantly colorful landscape. Croton Consumers Energy works with town- 8 ship, county, state and federal govern- ment officials, plus many volunteer Grand River PAGE organizations and private businesses to provide access to the clear water and Webber cool forests. 10 So grab the family, hop in the car and Kalamazoo River enjoy Michigan’s rivers and natural Allegan PAGE woodlands today. (Calkins Bridge) Let your family experience something a 10 little wild, but a lot wonderful. YOUR SAFETY IS A PRIORITY. See page 15 for tips. CONSUMERS ENERGY GET CLOSER TO NATURE • 3 MANISTEE RIVER Known locally as the “Big Manistee,” so as not to be confused with its smaller southern neighbor, the Little Manistee River, the Manistee River stretches about 170 miles from its headwaters near Alba to Manistee Lake and then Lake Michigan.