Drug Class Review on Newer Antiplatelet Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ticagrelor Antidote) by Mathematical Modeling
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Chalmers Publication Library Citation: CPT Pharmacometrics Syst. Pharmacol. (2016) 5, 313–323; doi:10.1002/psp4.12089 VC 2016 ASCPT All rights reserved ORIGINAL ARTICLE Unraveling the Pharmacokinetic Interaction of Ticagrelor and MEDI2452 (Ticagrelor Antidote) by Mathematical Modeling J Almquist1,2,3, M Penney4, S Pehrsson3, A-S Sandinge3, A Janefeldt3, S Maqbool4, S Madalli5, J Goodman4, S Nylander3 and P Gennemark3* The investigational ticagrelor-neutralizing antibody fragment, MEDI2452, is developed to rapidly and specifically reverse the antiplatelet effects of ticagrelor. However, the dynamic interaction of ticagrelor, the ticagrelor active metabolite (TAM), and MEDI2452, makes pharmacokinetic (PK) analysis nontrivial and mathematical modeling becomes essential to unravel the complex behavior of this system. We propose a mechanistic PK model, including a special observation model for post-sampling equilibration, which is validated and refined using mouse in vivo data from four studies of combined ticagrelor-MEDI2452 treatment. Model predictions of free ticagrelor and TAM plasma concentrations are subsequently used to drive a pharmacodynamic (PD) model that successfully describes platelet aggregation data. Furthermore, the model indicates that MEDI2452-bound ticagrelor is primarily eliminated together with MEDI2452 in the kidneys, and not recycled to the plasma, thereby providing a possible scenario for the extrapolation to humans. We anticipate the modeling work to improve PK and PD understanding, experimental design, and translational confidence. CPT Pharmacometrics Syst. Pharmacol. (2016) 5, 313–323; doi:10.1002/psp4.12089; published online 16 June 2016. Study Highlights WHAT IS THE CURRENT KNOWLEDGE ON THE WHAT THIS STUDY ADDS TO OUR KNOWLEDGE TOPIC? þ A mathematical model describing the simultaneous þ Antiplatelet therapy for the prevention of athero- PKs of ticagrelor and MEDI2452 in the mouse is pre- thrombotic events in patients with acute coronary syn- sented. -

Clopidogrel Compared with Other Antiplatelet Agents For
Canadian Agency for Agence canadienne Drugs and Technologies des médicaments et des in Health technologies de la santé CADTH Technology Report Issue 131 Clopidogrel Compared with Other Antiplatelet November 2010 Agents for Secondary Prevention of Vascular Events in Adults Undergoing Percutaneous Coronary Intervention: Clinical and Cost-Effectiveness Analyses Supporting Informed Decisions Until April 2006, the Canadian Agency for Drugs and Technologies in Health (CADTH) was known as the Canadian Coordinating Office for Health Technology Assessment (CCOHTA). Publications can be requested from: CADTH 600-865 Carling Avenue Ottawa ON Canada K1S 5S8 Tel: 613-226-2553 Fax: 613-226-5392 Email: [email protected] or downloaded from CADTH’s website: http://www.cadth.ca Cite as: Chen SY, Russell E, Banerjee S, Hutton B, Brown A, Asakawa K, McGahan L, Clark M, Severn M, Cox J, Sharma M. Clopidogrel Compared with Other Antiplatelet Agents for Secondary Prevention of Vascular Events in Adults Undergoing Percutaneous Coronary Intervention: Clinical and Cost-Effectiveness Analyses [Internet]. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2010 (Technology report; no. 131). [cited 2010-11-01]. Available from: http://www.cadth.ca/preview.php/en/publication/2708 Production of this report is made possible by financial contributions from Health Canada and the governments of Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland and Labrador, Northwest Territories, Nova Scotia, Nunavut, Prince Edward Island, Saskatchewan, and Yukon. The Canadian Agency for Drugs and Technologies in Health takes sole responsibility for the final form and content of this report. The views expressed herein do not necessarily represent the views of Health Canada or any provincial or territorial government. -

Multifactorial Background for a Low Biological Response to Antiplatelet Agents Used in Stroke Prevention
medicina Review Multifactorial Background for a Low Biological Response to Antiplatelet Agents Used in Stroke Prevention Adam Wi´sniewski Department of Neurology, Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toru´n, Skłodowskiej 9 Street, 85-094 Bydgoszcz, Poland; [email protected]; Tel.: +48-79-0813513 Abstract: Effective platelet inhibition is the main goal of the antiplatelet therapy recommended as a standard treatment in the secondary prevention of non-embolic ischemic stroke. Acetylsalicylic acid (aspirin) and clopidogrel are commonly used for this purpose worldwide. A low biological response to antiplatelet agents is a phenomenon that significantly reduces the therapeutic and protective properties of the therapy. The mechanisms leading to high on-treatment platelet reactivity are still unclear and remain multifactorial. The aim of the current review is to establish the background of resistance to antiplatelet agents commonly used in the secondary prevention of ischemic stroke and to explain the possible mechanisms. The most important factors influencing the incidence of a low biological response were demonstrated. The similarities and the differences in resistance to both drugs are emphasized, which may facilitate the selection of the appropriate antiplatelet agent in relation to specific clinical conditions and comorbidities. Despite the lack of indications for the routine assessment of platelet reactivity in stroke subjects, this should be performed in selected patients from the high-risk group. Increasing the detectability of low antiaggregant responders, in light of its negative impact on the prognosis and clinical outcomes, can contribute to a more individualized approach and modification of the antiplatelet therapy to maximize the therapeutic effect in the secondary prevention of stroke. -

Acute Coronary Syndrome
Abstracts Heart: first published as 10.1136/heartjnl-2011-300867.420 on 12 October 2011. Downloaded from Acute coronary syndrome http://heart.bmj.com/ [gw22-e0217] INFLUENCE OF RANITIDINE ON GASTROINTESTINAL HAEMORRHAGE AND THROMBOSIS INDUCED BY DUAL ANTIPLATELET THERAPY AFTER PERCUTANEOUS CORONARY INTERVENTION Zhangqiang Chen, Lang Hong, Hong Wang, Qiulin Yao, Heng Li Lai, Linxiang Lu Jiangxi Provincial People´s Hospital, Jiangxi, China on October 1, 2021 by guest. Protected copyright. 10.1136/heartjnl-2011-300867.420 Background Percutaneous coronary intervention (PCI) is an effective treatment. method of coronary heart disease, particularly acute coronary syndrome. A large number of evidence-based medicine has shown that patients with coro- nary heart disease after PCI obtain clinical signifi cant benefi t in conjunction with clopidogrel and aspirin therapy, while the most common side effects of antiplatelet drugs is of gastric injury, that can lead to gastric bleeding, Thus, the American Heart Association/American Gastroenterological Association/ American Heart Association guidelines proposed the patients for the acceptance of dual antiplatelet therapy were treated with proton pump inhibitor (PPI) to reduce gastrointestinal complications such as ulcers and bleeding risk in 2008. But recently, the observation of basic and clinical research suggests that proton pump inhibitor (PPI) can reduce clopidogrel inhibi- tory effect on platelet aggregation, increasing major adverse cardiac events (MACE). H2 receptor antagonist ranitidine do not pass cytochrome P450 metabolism, would such gastric mucosa protective agents affect the clopidogrel and aspirin Heart October 2011 Vol 97 Suppl 3 A143 Heartnjl.indd Sec1:143 10/4/2011 10:07:32 PM Abstracts Heart: first published as 10.1136/heartjnl-2011-300867.420 on 12 October 2011. -

Janus Kinase Inhibitors and Coronavirus Disease (COVID)-19: Rationale, Clinical Evidence and Safety Issues
pharmaceuticals Review Janus Kinase Inhibitors and Coronavirus Disease (COVID)-19: Rationale, Clinical Evidence and Safety Issues Milo Gatti 1,2,† , Eleonora Turrini 3,† , Emanuel Raschi 1,* , Piero Sestili 4 and Carmela Fimognari 3,* 1 Pharmacology Unit, Department of Medical and Surgical Sciences, Alma Mater Studiorum—Università di Bologna, Via Irnerio 48, 40126 Bologna, Italy; [email protected] 2 SSD Clinical Pharmacology, IRCCS Azienda Ospedaliero Universitaria Sant’Orsola, 40126 Bologna, Italy 3 Department for Life Quality Studies, Alma Mater Studiorum—Università di Bologna, C.so D’Augusto 237, 47921 Rimini, Italy; [email protected] 4 Department of Biomolecular Sciences (DISB), Università degli Studi di Urbino Carlo Bo, Via I Maggetti 26, 61029 Urbino, Italy; [email protected] * Correspondence: [email protected] (E.R.); carmela.fi[email protected] (C.F.) † These Authors contributed equally to this article. Abstract: We are witnessing a paradigm shift in drug development and clinical practice to fight the novel coronavirus disease (COVID-19), and a number of clinical trials have been or are being testing various pharmacological approaches to counteract viral load and its complications such as cytokine storm. However, data on the effectiveness of antiviral and immune therapies are still inconclusive and inconsistent. As compared to other candidate drugs to treat COVID-19, Janus Kinase (JAK) inhibitors, including baricitinib and ruxolitinib, possess key pharmacological features for a potentially successful Citation: -

Reduction in the Incidence of Myocardial Infarction in Patients with Rheumatoid Arthritis Who Respond to Anti–Tumor Necrosis Factor ␣ Therapy
View metadata, citation and similar papers at core.ac.uk brought to you by CORE ARTHRITIS & RHEUMATISM provided by PubMed Central Vol. 56, No. 9, September 2007, pp 2905–2912 DOI 10.1002/art.22809 © 2007, American College of Rheumatology Reduction in the Incidence of Myocardial Infarction in Patients With Rheumatoid Arthritis Who Respond to Anti–Tumor Necrosis Factor ␣ Therapy Results From the British Society for Rheumatology Biologics Register W. G. Dixon, K. D. Watson, M. Lunt, K. L. Hyrich, British Society for Rheumatology Biologics Register Control Centre Consortium, A. J. Silman, and D. P. M. Symmons, on behalf of the British Society for Rheumatology Biologics Register Objective. Rheumatoid arthritis (RA) is associ- rate of 4.8 events per 1,000 person-years and 5.9 events ated with an increased risk of coronary artery disease, per 1,000 person-years, respectively. After adjustment possibly acting via shared mechanisms of inflammation. for baseline risk factors, there was no reduction in the This study was undertaken to test the hypothesis that rate of MI in the anti-TNF␣ cohort compared with the the powerful antiinflammatory effect of anti–tumor DMARD cohort (incidence rate ratio 1.44 [95% confi- necrosis ␣ (anti-TNF␣) therapy might lead to a reduc- dence interval 0.56–3.67]). In an analysis of anti-TNF␣– tion in the incidence of myocardial infarction (MI) in treated patients who responded to the treatment within patients with RA. 6 months versus those who did not, MI rates were found Methods. Using data from the British Society for to be 3.5 events per 1,000 person-years in responders Rheumatology Biologics Register, a national prospective and 9.4 events per 1,000 person-years in nonresponders. -

Polypill Eligibility and Equivalent Intake in a Swiss Population-Based
www.nature.com/scientificreports OPEN Polypill eligibility and equivalent intake in a Swiss population‑based study Julien Castioni 1,2, Nazanin Abolhassani 1,2*, Peter Vollenweider 1, Gérard Waeber 1 & Pedro Marques‑Vidal 1 The polypill has been advocated for cardiovascular disease (CVD) management. The fraction of the population who could beneft from the polypill in Switzerland is unknown. Assess (1) the prevalence of subjects (a) eligible for the polypill and (b) already taking a polypill equivalent; and (2) the determinants of polypill intake in the frst (2009–2012) and second follow‑ups (2014–2017) of a population‑based prospective study conducted in Lausanne, Switzerland. The frst and the second follow‑ups included 5038 and 4596 participants aged 40–80 years, respectively. Polypill eligibility was defned as having a high CVD risk as assessed by an absolute CVD risk ≥ 5% with the SCORE equation for Switzerland and/or presenting with CVD. Four polypill equivalents were defned: statin + any antihypertensive with (A) or without (B) aspirin; statin + calcium channel blocker (CCB) (C); and statin + CCB + angiotensin‑converting enzyme inhibitor (D). The prevalence of polypill eligibility was 20.6% (95% CI 19.5–21.8) and 27.7% (26.5–29.1) in the frst and second follow‑up, respectively. However, only around one‑third of the eligible 29.5% (95% CI 26.7–32.3) and 30.4% (27.9–33.0) respectively, already took the polypill equivalents. All polypill equivalents were more prevalent among men, elderly and in presence of CVD. After multivariable adjustment, in both periods, male gender was associated with taking polypill equivalent A (OR: 1.93; 95% CI 1.45–2.55 and OR: 1.67; 95% CI 1.27–2.19, respectively) and polypill equivalent B (OR: 1.52; 95% CI 1.17–1.96 and OR: 1.41; 95% CI 1.07–1.85, respectively). -

Pharmacotherapy in Patients with Stable Coronary Artery Disease Treated on an Outpatient Basis in Poland
Original article Pharmacotherapy in patients with stable coronary artery disease treated on an outpatient basis in Poland. Results of the multicentre RECENT study Waldemar Banasiak1, Arleta Wilkins2, Robert Pociupany2, Piotr Ponikowski1 1 Heart Diseases Center, 4th Military Clinical Hospital, Wrocław, Poland 2 Servier Polska, Warsaw, Poland Abstract Background: Comprehension of therapeutic methods in patients with stable coronary artery disease (CAD) is mandatory for the introduction of successful prevention. Aim: To gather information regarding individuals with stable coronary artery disease treated by specialists and general practitioners on an outpatient basis. Methods: A representative group of 215 general practitioners and 67 specialists participated in this study. The analysis contains data concerning pharmacotherapy in a group of 2,593 patients with stable CAD (mean age – 65.0±9.8 years, women – 44.6%). Results: The patients received the following treatment: acetylsalicylic acid – 75.3%, other antiplatelet drugs – 6.6% (antiplatelet drugs altogether – 81.9%), beta-blockers – 81.1%, ACE-I – 78.8%, statins – 71.9%, fibrates – 4.7%, long-acting nitrates – 53.0%, short- -acting nitrates – 33.1%, molsidomine – 18.2%, calcium channel blockers – 23.8%, metabolic drugs (trimetazidine) – 13.4%, diuretics – 43.5%, and angiotensin receptor antagonists – 1.7% of patients. Drugs classified as non-cardiological were received by 36.2% of patients. The optimal pharmacotherapy including four medications, one from each therapeutic class used in order to improve the prognosis of the patient (an antiplatelet drug, a beta-blocker, an ACE-I, statin), was received by a total of 45.8% of the participants, three medications by 31.7%, two medications by 15.8%, and one medication by 5.5%; 1.2% of the participants did not receive any medication from the four groups of drugs improving prognosis. -

Evaluation of the Antiplatelet Effects of Cilostazol, a Phosphodiesterase 3 Inhibitor, by VASP Phosphorylation and Platelet Aggregation
Circ J 2008; 72: 1844–1851 Evaluation of the Antiplatelet Effects of Cilostazol, a Phosphodiesterase 3 Inhibitor, by VASP Phosphorylation and Platelet Aggregation Hiromi Yamamoto, MD; Kanako Takahashi, MT; Haruyo Watanabe, MT; Yuka Yoshikawa, MT; Ryutaro Shirakawa, PhD; Tomohito Higashi, PhD; Mitsunori Kawato, MD; Tomoyuki Ikeda, MD; Arata Tabuchi, MD**; Takeshi Morimoto, MD*; Toru Kita, MD; Hisanori Horiuchi, MD Background Cilostazol, a phosphodiesterase 3 inhibitor, is an antiplatelet drug that is widely used for prevent- ing cardiovascular events, although, to date, there are few methods for evaluating its effects. Methods and Results Blood samples were taken at baseline and at 3 and 12h in 10 healthy male subjects after 100mg cilostazol intake. Each sample was examined by Western blot for phosphorylation levels of vasodilator- stimulated phosphoprotein (VASP), an abundant cAMP-dependent kinase substrate in platelets, and by the optical aggregometer for ADP- and collagen-induced aggregation, before and after 8nmol/L prostaglandin E1 (PGE1) treatment. Cilostazol intake did not affect VASP phosphorylation levels or the maximal aggregation rates without PGE1 treatment. However, cilostazol intake apparently enhanced PGE1-induced VASP phosphorylation and PGE1- mediated reduction of ADP- and collagen-induced maximal aggregation rates. Levels of VASP phosphorylated at Ser157 were correlated and the maximal aggregation rates induced by ADP were inversely correlated with cilostazol concentrations in the plasma. Conclusion The antiplatelet effects -

PRIMIS Making Clinical Data Work
WHAT DID WE ACHIEVE IN WESSEX? Overview • Action learning set delivered in April 2016 with follow up PINCER training in June 2016 • 229 licences were paid for by the AHSN and made available to practices as follows: April 2016 - Fareham and Gosport practices May 2016 - Dorset, Portsmouth and South East Hampshire practices June 2016 - North East Hampshire and Farnham, North Hampshire and Southampton practices • To date 208 practices have downloaded PINCER since the beginning of the project • To date 142 practices have shared their aggregate data since the beginning of this project • Licences were extended so that all practices had at least 12 months use and re-purchased by the AHSN for 156 practices Cumulative figures over 4 quarters No of practices downloading % uptake Dorset 88 90% Fareham and Gosport 15 71% Portsmouth 17 81% North East Hampshire andCumulative Farnham figures over 4 quarters 21 88% South Eastern Hampshire 18 75% North Hampshire 19 95% Southampton 30 94% Total 208 87% • So we know that there are plenty of you using the tool – unless you have just downloaded it and walked away from the PC! • The next requirement in the project was to upload baseline data to CHART Online • 142 out of 208 practices have shared aggregate baseline data Practices downloading vs uploading 120 100 80 60 40 20 0 Dorset Fareham and Portsmouth North East South Eastern North Hampshire Southampton Gosport Hampshire and Hampshire Farnham Download Upload Initial analysis • Of the 142 practices that shared their baseline data, 126 practices uploaded data -

New Blood-Thinner Zontivity: Expanded Comparative Safety Research Profile
For more information contact us New Blood-Thinner Zontivity: Expanded Comparative Safety Research Profile © Copyright. 2015 Advera Health Analytics, Inc. All rights reserved. This material MAY NOT BE REPRODUCED, DISPLAYED, MODIFIED, DISTRIBUTED or LINKED TO without the express prior written permission of the copyright holder. Advera Health Analytics Inc’s research may be cited but not excerpted in its entirety. For permission, contact Sharon Miller Drugs Covered: BRILINTA, EFFIENT, PLAVIX, heart in patients with previous myocardial infarction (heart ZONTIVITY attack) or blockages in the arteries to the legs. Zontivity is contraindicated for use in patients with a history of stroke Classes Covered: Platelet aggregation inhibitors excl. or transient ischemic attack (TIA), as there was substantially heparin increased risk of intracranial hemorrhage (ICH) paired with Indications Covered: Myocardial Infarction, Stroke, no apparent benefit of treatment in this subset of patients in Thromboembolic Events Zontivity’s clinical trial. Mechanism of Action Covered: Thrombin Inhibitors Like other antiplatelets (e.g. Brilinta, Effient, Plavix), Zontivity has been shown to increase patient risk for potentially fatal bleeding, which has been reflected in Actionable Intelligence: AdverseEvents, Inc. (AEI) the Boxed Warning included in the drug’s prescribing analyzed Zontivity (vorpaxar, Merck), the newly approved information. The use of this drug is restricted to certain high- antiplatelet agent indicated for the reduction of heart attack, risk patient populations due to its proclivity for inducing stroke, and CV death in patients with history of heart attack serious bleeding and intracranial hemorrhage (ICH). or blockages in the arteries to the legs. We compared the existing safety profile of Zontivity to the antiplatelet agents Zontivity is the first FDA-approved drug in the class of Brilinta (ticagrelor), Plavix (clopidogrel), and Effient protease-activated receptor-1 (PAR-1) antagonists, and (prasugrel). -
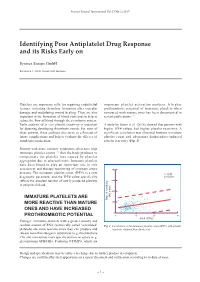
Identifying Poor Antiplatelet Drug Response and Its Risks Early On
Sysmex Journal International Vol.27 No.2 (2017) Identifying Poor Antiplatelet Drug Response and its Risks Early on Sysmex Europe GmbH Bornbarch 1, 22848 Norderstedt, Germany Platelets are important cells for repairing endothelial important platelet activation markers. A higher lesions, initiating thrombus formation after vascular prothrombotic potential of immature platelets when damage and modulating wound healing. They are also compared with mature ones has been documented in important in the formation of blood clots and so help to several publications 3-6). reduce the flow of blood through the circulatory system. Early analysis of in vivo platelet reactivity is important A study by Stratz et al. (2016) showed that patients with for detecting developing thrombotic events. For most of higher IPF# values had higher platelet reactivity. A these patients, these analyses also serve as a forecast of significant correlation was observed between immature future complications and help to evaluate the efficacy of platelet count and adenosine diphosphate-induced antiplatelet medication. platelet reactivity (Fig. 1) 5). Patients with acute coronary syndromes often have high immature platelet counts 1, 2) that the body produces to compensate for platelet loss caused by platelet aggregation due to atherosclerosis. Immature platelets have been found to play an important role in risk assessment and therapy monitoring of coronary artery 800 diseases. The immature platelet count (IPF#) is a new r = 0.26 diagnostic parameter and the IPF# value specifically p < 0.001 reflects the absolute number of newly produced platelets 600 in peripheral blood. 400 (AU x min) IMMATURE PLATELETS ARE Platelet reactivity MORE REACTIVE THAN MATURE 200 ONES AND HAVE INCREASED 0 PROTHROMBOTIC POTENTIAL 0 10 20 30 IPF# (109/L) Younger, immature platelets with a greater density and residual amount of RNA (historically called ‘reticulated’ Fig.