Oxygen Generation and Storage: Oxygen Concentrator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Frequently Asked Questions (Faqs)
Frequently Asked Questions (FAQs) What is hyperbaric oxygen therapy? Commonly referred to as HBOT, hyperbaric oxygen therapy enhances the body’s natural healing process by delivering oxygen under pressure, which increases the oxygen content in the blood, plasma, cerebral spinal fluid, and other body tissues. There are two basic types of HBOT—hard HBOT and mild HBOT. With hard HBOT, treatments are delivered in a hard-sided chamber typically at pressures greater than 1.5 ATA and using 100% oxygen. 100% oxygen is extremely flammable; therefore, hard HBOT involves managing the risk of explosion. Another concern with hard HBOT is oxygen toxicity. While hard HBOT with 100% oxygen results in greater oxygen saturation in the tissues, many conditions respond better to mild HBOT. In clinical trials to date, there has been virtually no difference in clinical outcome between mild HBOT and hard HBOT. Mild HBOT refers to hyperbaric oxygen therapy at lower pressures, typically 1.5 ATA or below, and the use of an oxygen concentrator delivering 90-95% oxygen inside a portable soft-sided chamber. Mild HBOT has no known safety risks with fire or toxicity, and it is substantially less expensive. Our facility provides concentrated oxygen (90-95%) at 1.3 ATA—(Mild HBOT)a highly effective combination clinically, and without the risk of oxygen toxicity or explosion, as 100% oxygen is avoided. How should I expect mild HBOT to feel? You will be seated or lying down inside the chamber, relaxing comfortably in your own clothing, as you breathe concentrated oxygen (90-95% O2) through a facemask. -

Stratus 5 Oxygen Concentrator User Manual
USER MANUAL OXYGEN CONCENTRATOR CE 0123 v2.1 STR1005 User Manual Symbol Key MARK DEFINITION II Power on Power off Follow Instruction for Use No smoking Caution, consult accompanying documents. Class Ⅱ (Double Insulated) Type BF Applied Part CE certification mark 0123 AC Power Stacking Limit by Number This Way Up Fragile, handle with care Keep dry Temperature limit No open flames IIPP2211 IP21 Drip Proof Equipment Consult instructions for use Stand-by Warning, electricity 2 v2.1 STR1005 User Manual 3 v2.1 STR1005 User Manual SPECIAL NOTES • Please read this manual carefully before using this product and save it for future reference. • If you need assistance with this manual, Please contact your local DME or home health provider • The Stratus 5 is a prescription device. Use only the liter setting prescribed for you. • It is always recommended for critically ill patients to have a backup oxygen source in case of malfunction. • If patient experiences an adverse reaction contact physician or call 911 immediately. • In case of machine malfunction, contact the home medical equipment provider; do not attempt to disassemble the Stratus 5. • The Stratus 5 is not intended as life support, it is for supplemental oxygen use only. Patients with special needs may be unable to understand the alarm features and should be well supervised while using an oxygen concentrator. • The Stratus 5 is for single patient use. • Do not adjust the flowmeter float beyond the red line position. Long-term use out of range will reduce the efficiency of the oxygen generator. SAFETY NOTICE Please read the following information carefully before Operating the oxygen concentrator Warning Special attention should be paid to reducing the risk of fire when using oxygen therapy. -
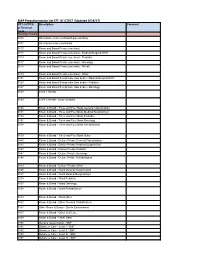
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17)
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17) CPT, HCPCS Description Comment or Revenue Code Revenue Codes 0100 All inclusive room and board plus ancillary 0101 All inclusive room and board 0110 Room and Board Private (one bed) 0111 Room and Board Private (one bed) - Medical/Surgical/GYN 0113 Room and Board Private (one bed) - Pediatric 0117 Room and Board Private (one bed) - Oncology 0118 Room and Board Private (one bed) - Rehab 0119 Room and Board Private (one bed) - Other 0121 Room and Board Semiprivate (two beds) - Medical/Surgical/GYN 0123 Room and Board Semiprivate (two beds) - Pediatric 0127 Room and Board Semiprivate (two beds) - Oncology 0128 Level 1 Rehab 0129 Level 2 Rehab - acute complex 0130 Room & Board - Three and Four Beds General Classification 0131 Room & Board - Three and Four Beds Medical/Surgical/Gyn 0133 Room & Board - Three and Four Beds Pediatric 0137 Room & Board - Three and Four Beds Oncology 0138 Room & Board - Three and Four Beds Rehabilitation 0139 Room & Board - Three and Four Beds Other 0140 Room & Board - Deluxe Private General Classification 0141 Room & Board - Deluxe Private Medical/Surgical/Gyn 0143 Room & Board - Deluxe Private Pediatric 0147 Room & Board - Deluxe Private Oncology 0148 Room & Board - Deluxe Private Rehabilitation 0149 Room & Board - Deluxe Private Other 0150 Room & Board - Ward General Classification 0151 Room & Board - Ward Medical/Surgical/Gyn 0153 Room & Board - Ward Pediatric 0157 Room & Board - Ward Oncology 0158 Room & Board - Ward Rehabilitation 0159 Room & Board - -

E-Cigarette Use in Patients Receiving Home Oxygen Therapy
FOCUSED REVIEW E-cigarette use in patients receiving home oxygen therapy Yves Lacasse MD MSc FRCP1,2, Martin Légaré MD FRCP3, François Maltais MD FRCP1,2 Y Lacasse, M Légaré, F Maltais. E-cigarette use in patients receiving La cigarette électronique chez les patients sous home oxygen therapy. Can Respir J 2015;22(2):83-85. oxygénothérapie à domicile Current smokers who are prescribed home oxygen may not benefit from the therapy. In addition to being an obvious fire hazard, there is some evi- Il se peut que les fumeurs qui se font prescrire une oxygénothérapie à domi- dence that the physiological mechanisms by which home oxygen is cile ne profitent pas de ce traitement. Sans compter que le tabagisme pose believed to operate are inhibited by smoking. Although their effectiveness un risque d’incendie évident, certaines données probantes indiquent qu’il is yet to be demonstrated, electronic cigarettes (e-cigarettes) are often inhibe les mécanismes physiologiques par lesquels l’oxygénothérapie à regarded as an aid to smoking cessation. However, several burn accidents domicile fonctionnerait. Même si son efficacité reste à démontrer, la ciga- in e-cigarette smokers receiving home oxygen therapy have also been rette électronique (vapoteuse) est souvent perçue comme une aide au reported, leading Health Canada to release a warning of fire risk to oxygen sevrage du tabagisme. Cependant, plusieurs incidents de brûlure chez des therapy patients from e-cigarettes. It is the authors’ position that patients vapoteurs sous oxygénothérapie à domicile ont été déclarés, ce qui a incité receiving oxygen should definitely not use e-cigarettes. -

Florida Special Needs Registry Registration Information - Osceola County
Florida Special Needs Registry Registration Information - Osceola County Instructions: Complete this form and fax or mail it to Osceola County to register an individual for the Florida Special Needs Registry. This form is not required if you have already registered on line. Required fields are indicated with an asterisk (*). Mail: Osceola County Special Needs Registry Fax: (407) 742-9022 2586 Partin Settlement Road Kissimmee, FL 34744 PERSONAL INFORMATION ABOUT THE REGISTRANT *First Name Middle Name *Last Name Suffix *Birth Date *Gender (select only one) Male Female Transgender Non-Binary Prefer Not To Provide *Height Feet: Inches: *Weight (pounds) Living Situation (select only one) Live alone Live with relative or Other living situation caregiver *Primary Language Secondary Language Veteran Yes No Last 4 digits of SSN Email Address Are you completing this form on behalf of the Family Member Caregiver Neighbor Friend registrant? If so, please indicate your relationship to the registrant (select only Home Health Care County Emergency County Health DOH State Staff one) Provider Management Staff Department Staff ADDRESS FOR THE REGISTRANT (physical address is required) *Physical Address (cannot be a PO Box) *Physical City *Physical State FL *Physical Zip Code Name of Complex, Subdivision or Mobile Home Park Is the home at this address a mobile home? Yes No Is the home at this address a highrise or Yes No multi-story home? Does this home have stairs? Yes No Is there a gate that requires a code to enter? Yes No Do you live at this address -

Emblemhealth's Pre-Authorization List
EmblemHealth's Pre-authorization List This is a complete list of all services requiring a Prior Approval for HIP members or a Pre-Certification for GHI members* (jointly referred to as "pre-authorization") subject to their benefit plan's coverage for all places of service, including Office (POS 11). The list accounts for EmblemHealth's medical policies, medical technology database, provider manual, and special utilization management programs. Pre-authorization is not a guarantee of payment. Payment is subject to a member's eligibility for benefits on the date of service. Emergency services do not require a pre-authorization. *GHI PPO City of New York employees and non-Medicare eligible retirees with GHI PPO benefits will be managed by Empire BCBS for inpatient and outpatient services. To see what needs authorization, use their look-up tool: https://www.empireblue.com/wps/portal/ehpprovider. CPT/ HCPCS Description Code 0008M Oncology (breast), mRNA analysis of 58 genes using hybrid capture, on formalin-fixed paraffin- embedded (FFPE) tissue, prognostic algorithm reported as a risk score 00640 Anesthesia for manipulation of the spine or for closed procedures on the cervical, thoracic or lumbar spine 0085T Breath test for heart transplant rejection 0100T Placement of a subconjunctival retinal prosthesis receiver and pulse generator, and implantation of intra-ocular retinal electrode array, with vitrectomy 0195T Arthrodesis, pre-sacral interbody technique, disc space preparation, discectomy, without instrumentation, with image guidance, includes -

Portable Oxygen Concentrator Therapy Guide Guidance for Patients Requiring Long-Term, Supplemental Oxygen Solutions
Portable Oxygen Concentrator Therapy Guide Guidance for patients requiring long-term, supplemental oxygen solutions. Table of Contents Part One: Oxygen Therapy 101 Part Two: Oxygen Concentrators Part Three: Portable Oxygen Concentrators Part Four: Which Oxygen Concentrator is Right For You? Confused or overwhelmed? Let us help you! Our Product Specialists are standing by to answer questions and offer tips.(800) 515-8049 INTRODUCTION 3 Welcome! OxygenDirect is pleased to provide this educational guide for current and potential patients to understand the benefits of oxygen therapy—and specifically portable oxygen concentrators. We intend to expand patients’ knowledge and appreciation of advancements in technology that are now available. This document is designed to complement the information provided by a doctor or healthcare provider when discussing oxygen therapy options. Who is OxygenDirect? OxygenDirect is dedicated to helping oxygen therapy patients breathe easier without interfering with lifestyles and daily activities. We offer a wide selection of portable and home oxygen products and accessories, plus a diverse range of CPAP machines and accessories. Our OxygenDirect Product Specialists can help evaluate patients’ oxygen needs and varying product features to ultimately arrive at a product that perfectly fits a wide range of lifestyles and budgets. Disclaimer: The information contained in this document is general in nature and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health providers with any questions you may have regarding a breathing/medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this document. -

Oxygen Safety
Oxygen Safety Table of Contents Page In your Home 1. Prepare your Home ..................................................................... 3 2. Fire Safety Guidelines................................................................. 4 3. Oxygen Storage and Handling ................................................... 6 4. Concentrator Safety .................................................................... 6 5. Liquid Oxygen Safety .................................................................. 6 On the Go 6. Tips for Traveling with Oxygen .................................................. 7 7. Automobile Travel with Oxygen ................................................. 7 8. Cruise Line Travel with Oxygen ................................................. 8 9. Bus Travel with Oxygen .............................................................. 8 10. Train Travel with Oxygen ............................................................ 9 11. Airline Travel with Oxygen ......................................................... 9 References .................................................................................................. 11 Contact Us .................................................................................................. 11 Printable No Smoking Sign ....................................................................... 12 Printable Oxygen in Use Sign ................................................................... 13 This booklet provides general safety information for oxygen concentrators, compressed -

Breathing BETTER a Handbook for People with COPD and Other Chronic Lung Conditions ©2010-2015 Intermountain Healthcare
Breathing BETTER A handbook for people with COPD and other chronic lung conditions ©2010-2015 Intermountain Healthcare. This booklet can help you learn how to breathe better, do more, and live well with lung disease. 2 What’s inside… About COPD and Other Chronic Lung Diseases........page 4 Normal lungs and breathing...............................................4 Breathing with COPD........................................................5 Other chronic lung conditions.............................................6 Assessment and Treatment Overview.....................page 8 Initial assessment.............................................................8 Lung function tests..........................................................9 Treatments for lung disease................................................9 Quitting smoking.............................................................10 Pulmonary rehabilitation....................................................10 Medication....................................................................page 11 Medication basics..........................................................11 Medication types...........................................................12 Medication delivery devices...............................................14 How to use an MDI (metered-dose inhaler)...........................15 How to use a DPI (dry-powder inhaler).................................17 How to use a nebulizer....................................................19 Oxygen Therapy............................................................page -

Firstcare STAR Prior Authorization List Effective January 1, 2021
FirstCare STAR Prior Authorization List Effective January 1, 2021 FirstCare Health Plans STAR Medicaid in-network providers are encouraged to log in1 to the Provider Portal to verify member eligibility2 status and utilize the Authorization Code Look-up to submit new authorization requests, view authorization status, and view prior authorization requirements. Alternately, complete the Essential Information to Initiate an Authorization3 on the FirstCare Authorization Request Form and submit the Complete Authorization Request4 via fax. PRESS "CTRL" AND "F" KEYS AT THE SAME TIME TO BRING UP THE SEARCH BOX. ENTER A PROCEDURE CODE OR DESCRIPTION OF THE SERVICE. 5 Effective Prior Authorization Medical Policy Date Prior Authorization is required for ALL SERVICES provided by NON-CONTRACTED providers except for use of out- 261 01/21/13 of-network benefits in PPO and POS products, unless required per listing below6 Notification required for admission to these facilities/services and will be subject to admission review concurrent review:7 1/21/13 1. Contracted hospitals for medical, surgical, and behavioral health services 1. MCG5, 250 2. Contracted hospice programs (applies to inpatient and outpatient programs) 2. MCG5 Notification required for DISCHARGE from all facilities n/a 01/21/13 Prior Authorization required for admission to facilities/programs listed below: 5 1. Long-term Acute Care (LTAC) hospitals, 1. MCG 01/21/13 2. Inpatient Rehabilitation hospitals 2. MN-248 3. Behavioral health/substance abuse residential, partial hospitalization, -

HOME OXYGEN FIRE SAFETY a Fact Sheet for Home Oxygen Users
New York State Office of Fire Prevention and Control HOME OXYGEN FIRE SAFETY A Fact Sheet for Home Oxygen Users This bulletin provides general safety information for oxygen equipment used for medical purposes in private homes, apartments and condominiums. Oxygen Liquid oxygen @Do not use liquid oxygen vessels that exceed 10 gallons. Oxygen is not flammable, but it can Oxygen is stored as a very cold cause other materials that burn to liquid in a vessel very similar to a Safety thermos. When released, the liquid ignite more easily and to burn far @Never smoke while using oxygen. more rapidly. The result is that a converts to a gas and you breathe it fire involving oxygen can appear in just like compressed gas. This @Warn visitors not to smoke near storage method takes up less space you when you are using oxygen. explosive-like. Oxygen is of great benefit to those in need of than the compressed gas cylinder, @Post at least one NO SMOKING respiratory therapy but it should and you can transfer the liquid to a sign in a prominent place at the always be handled with caution and small, portable vessel at home. entrance to your home. Liquid oxygen is more expensive awareness of the potential hazards. @ than the compressed gas and the When you go to a restaurant with The Equipment vessel vents when it is not in use. your portable oxygen source, sit There are three common ways of An oxygen-conserving device may in the nonsmoking section and providing oxygen therapy. Oxygen be built into the vessel to conserve away from any open flame such can be delivered to your home in as candles or warming burners. -

Recent Advances and Technique in Anaesthesia for Ss/Js in Raj. State Health System
Training module Recent Advances And Technique In Anaesthesia For Ss/Js In Raj. State Health System. INDEX SESSION / SERIAL TOPIC PAGE NO. minute NO. 1. PROGRAMME 3 2. AIMS AND OBJECTIVE 4 DAY1 -a A. ANAESTHESIA MACHINE, EQUIPMENT AND TECHNIQUES 1a -30m 3. EXPERIENCE WITH THE GLOSVENT ANAESTHETIC 5-7 MACHINE 1b -30m 4. VAPORISERS 8 1c -30m 5. POSITIONING ON THE OPERARING TABLE 9-12 DAY1 -b B.PHARMACOLOGY 2a -45 m 6. NITRIC OXIDE AND PROPOFOL 13 -15 2b -45 m 7. LEVOBUPIVACAINE 16 -18 3-90m 8. ACUTE OXYGEN TREATMENT 19 -26 4a -45m 9. A NEW MANDATE FOR THE ANAESTHESIOLOGIST - 27 -32 CANCER PAIN SPECIALIST:EXPERTISE IN PRESCRIBING ANALGESICS. 4b -45m 10 EMERGENCY DRUGS IN ANAESTHESIOLOGY AND 33-34 CRITICAL CARE MEDICINE DAY2 C. ANAESTHESIA IN PATIENTS ASSOCIATED WITH COMORBID CONDITIONS 1-90 m 20 MONITERING DURING CAESAREAN SECTION 35-38 2a -45m 11. THR PATIENT WITH HEART DISEASE 39-42 2b -45m 12. ANAESTHESIA AND CHRONIC RENAL FAILURE 43 -45 3a -45m 13 ANESTHESIA AND LIVER DISEASE 46 -48 4-90m 14 CLINICAL MANAGEMENT OF DIABETES MELLITUS 49 -56 DURING ANAESTHESIA AND SURGERY 3b -45m 15 ANAESTHESIA FOR THE PATIENT WITH 57 -58 RESPIRATORY DISEASE DAY3 D .ANAESTHESIA - SPECIAL COSIDER ATION 1a -30m 16 A MOBILE ANAESTHESIA SERVICE 59 1b -60m 17 A PRACTICAL APPROACH TO EMERGENCY EYE 60 -64 ANAESTHESIA 2-90m 18 ANAESTHESIA FOR ELDERLY PATIENT 65 -69 3-90m 19 ANAESTHESIA FOR THE PATIENT REQUIRING 70 -83 EMERGENCY ABDOMINAL SURGER Y 4-90m 20 INDUCTION OF ANAESTHESIA IN PAEDIATRIC 84 -88 PATIENTS DAY4 F.