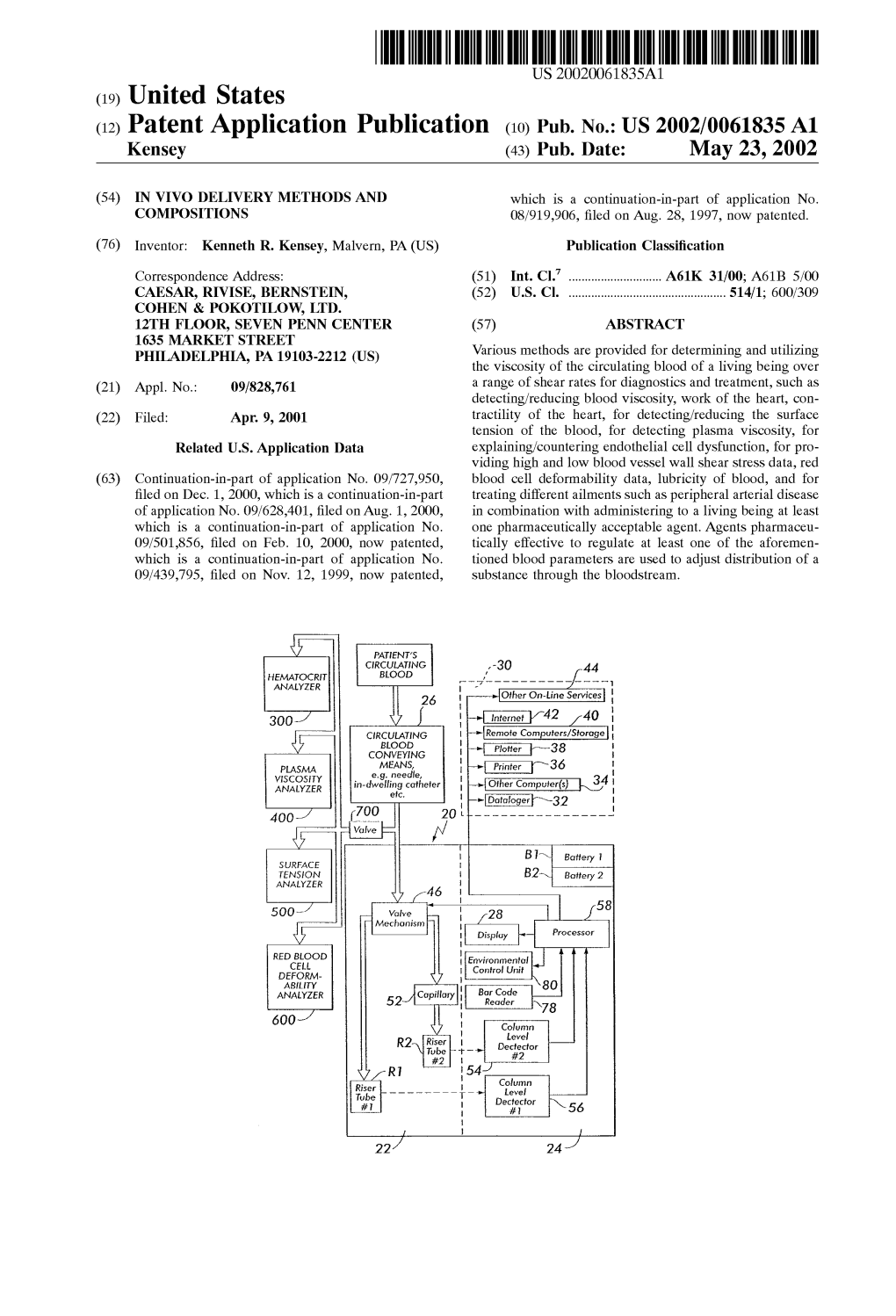
(12) Patent Application Publication (10) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protective Role of Parnaparin in Reducing Systemic Inflammation and Atherosclerotic Plaque Formation in Apoe-/- Mice
INTERNATIONAL JOURNAL OF MoleCular MEDICine 27: 561-565, 2011 Protective role of parnaparin in reducing systemic inflammation and atherosclerotic plaque formation in ApoE-/- mice MARCO ArtiCO1, RACHELE RIGANÒ2, BRiGitta Buttari2, ELisabetta PROFUMO2, BRUNELLA Ionta1, sANDRO BOsCO3, MANUELA RASILE2, ENRiCA BiANCHi1, MOiRA BRUNO1 and LORENZO FUMAGALLI4 1Department of sensory Organs, University of Rome ‘sapienza’; 2Department of Infectious, Parasitic and Immuno-mediated Diseases of the superior Health institute of Rome; Departments of 3Experimental Medicine and 4Anatomical, Histological, Medico-legal and Locomotor system sciences, University of Rome ‘sapienza’, Rome, italy Received November 15, 2010; Accepted December 29, 2010 DOi: 10.3892/ijmm.2011.606 Abstract. Atherosclerosis is a degenerative disease whose Unstable atherosclerotic plaques are vulnerable to rupture, and role in the onset and development of cardiovascular patholo- usually contain an increased accumulation of inflammatory gies and complications is of importance. Due to its silent but cells, particularly macrophages and T-lymphocytes secreting progressive development, and considering the endothelial, growth factors (EGF and bFGF) and pro-inflammatory immunological and inflammatory processes that are involved cytokines (4-6). Rather than remaining localized within in its clinical course, this still relatively unknown pathological the ‘culprit’ plaque, the inflammatory components spread condition has been and continues to be a matter of investiga- throughout the peripheral blood. Several studies suggest an tion worldwide. Our experience with previous studies on association between pro-inflammatory cytokines (TNFα, IL-6, atherosclerosis led us to investigate the possible influence IL-1) in the peripheral blood of patients with atherosclerosis of a low molecular weight heparin (LMWH) – Parnaparin® and the clinical outcome or stage of atherosclerotic disease on the development and clinical course of atherosclerosis in (7-11). -

Ovid MEDLINE(R)
Supplementary material BMJ Open Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily <1946 to September 16, 2019> # Searches Results 1 exp Hypertension/ 247434 2 hypertens*.tw,kf. 420857 3 ((high* or elevat* or greater* or control*) adj4 (blood or systolic or diastolic) adj4 68657 pressure*).tw,kf. 4 1 or 2 or 3 501365 5 Sex Characteristics/ 52287 6 Sex/ 7632 7 Sex ratio/ 9049 8 Sex Factors/ 254781 9 ((sex* or gender* or man or men or male* or woman or women or female*) adj3 336361 (difference* or different or characteristic* or ratio* or factor* or imbalanc* or issue* or specific* or disparit* or dependen* or dimorphism* or gap or gaps or influenc* or discrepan* or distribut* or composition*)).tw,kf. 10 or/5-9 559186 11 4 and 10 24653 12 exp Antihypertensive Agents/ 254343 13 (antihypertensiv* or anti-hypertensiv* or ((anti?hyperten* or anti-hyperten*) adj5 52111 (therap* or treat* or effective*))).tw,kf. 14 Calcium Channel Blockers/ 36287 15 (calcium adj2 (channel* or exogenous*) adj2 (block* or inhibitor* or 20534 antagonist*)).tw,kf. 16 (agatoxin or amlodipine or anipamil or aranidipine or atagabalin or azelnidipine or 86627 azidodiltiazem or azidopamil or azidopine or belfosdil or benidipine or bepridil or brinazarone or calciseptine or caroverine or cilnidipine or clentiazem or clevidipine or columbianadin or conotoxin or cronidipine or darodipine or deacetyl n nordiltiazem or deacetyl n o dinordiltiazem or deacetyl o nordiltiazem or deacetyldiltiazem or dealkylnorverapamil or dealkylverapamil -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

(12) Patent Application Publication (10) Pub. No.: US 2010/0209499 A1 Cumming Et Al
US 2010O209499A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0209499 A1 Cumming et al. (43) Pub. Date: Aug. 19, 2010 (54) SOLID ORAL DOSAGE FORM CONTAINING A 6LX 3L/727 (2006.01) AN ENHANCER A638/24 (2006.01) A638/28 (2006.01) (76) Inventors: Kenneth I. Cumming, Dublin (IE): A638/08 (2006.01) Zebunnissa Ramtoola, Dublin (IE) A6II 3/66 (2006.01) A638/3 (2006.01) Correspondence Address: A6IR 9/22 (2006.01) MYERS BIGELSIBLEY & SAJOVEC A 6LX 9/52 (2006.01) POBOX37428 A6IP 43/00 (2006.01) RALEIGH, NC 27627 (US) (52) U.S. Cl. ............. 424/456; 514/784: 514/54: 514/12: (21) Appl. No.: 12/768,008 514/2: 514/56; 514/8: 514/3: 514/15: 514/108; 514/11; 424/468; 424/457 (22) Filed: Apr. 27, 2010 (57) ABSTRACT Related U.S. Application Data The invention relates to a solid oral dosage form comprising (63) Continuation of application No. 12/172,707, filed on a pharmaceutically active ingredient in combination with an Jul. 14, 2008. enhancer which enhances the bioavailability and/or the absorption of the active ingredient. Accordingly, a Solid oral Publication Classification dosage form comprises a drug and an enhancer wherein the (51) Int. C. enhancer is a medium chain fatty acid ester, ether or salt or a A6 IK 9/64 (2006.01) derivative of a medium chain fatty acid, which is, preferably, A6 IK 47/12 (2006.01) Solid at room temperature and which has a carbon chain A 6LX 3L/75 (2006.01) length of from 6 to 20 carbonatoms. -

Drug Delivery System for Use in the Treatment Or Diagnosis of Neurological Disorders
(19) TZZ __T (11) EP 2 774 991 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 10.09.2014 Bulletin 2014/37 C12N 15/86 (2006.01) A61K 48/00 (2006.01) (21) Application number: 13001491.3 (22) Date of filing: 22.03.2013 (84) Designated Contracting States: • Manninga, Heiko AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 37073 Göttingen (DE) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO •Götzke,Armin PL PT RO RS SE SI SK SM TR 97070 Würzburg (DE) Designated Extension States: • Glassmann, Alexander BA ME 50999 Köln (DE) (30) Priority: 06.03.2013 PCT/EP2013/000656 (74) Representative: von Renesse, Dorothea et al König-Szynka-Tilmann-von Renesse (71) Applicant: Life Science Inkubator Betriebs GmbH Patentanwälte Partnerschaft mbB & Co. KG Postfach 11 09 46 53175 Bonn (DE) 40509 Düsseldorf (DE) (72) Inventors: • Demina, Victoria 53175 Bonn (DE) (54) Drug delivery system for use in the treatment or diagnosis of neurological disorders (57) The invention relates to VLP derived from poly- ment or diagnosis of a neurological disease, in particular oma virus loaded with a drug (cargo) as a drug delivery multiple sclerosis, Parkinsons’s disease or Alzheimer’s system for transporting said drug into the CNS for treat- disease. EP 2 774 991 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 774 991 A1 Description FIELD OF THE INVENTION 5 [0001] The invention relates to the use of virus like particles (VLP) of the type of human polyoma virus for use as drug delivery system for the treatment or diagnosis of neurological disorders. -

Composition Modulating Botulinum Neurotoxin Effect
(19) *EP003753568A1* (11) EP 3 753 568 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 23.12.2020 Bulletin 2020/52 A61K 38/17 (2006.01) A61K 31/205 (2006.01) A61K 31/4178 (2006.01) A61K 38/48 (2006.01) (2006.01) (2006.01) (21) Application number: 19181635.4 A61P 9/00 A61P 21/00 A61P 29/00 (2006.01) (22) Date of filing: 21.06.2019 (84) Designated Contracting States: •Cros, Cécile AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 74580 Viry (FR) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • Hulo, Nicolas PL PT RO RS SE SI SK SM TR 1252 Meinier (CH) Designated Extension States: • Machicoane, Mickaël BA ME 1290 Versoix (CH) Designated Validation States: KH MA MD TN (74) Representative: Barbot, Willy Simodoro-ip (71) Applicant: Fastox Pharma SA 82, rue Sylvabelle 1003 Lausanne (CH) 13006 Marseille (FR) (72) Inventors: • Le Doussal, Jean-Marc 1007 Lausanne (CH) (54) COMPOSITION MODULATING BOTULINUM NEUROTOXIN EFFECT (57) The present invention relates to a method for cholinergic neuronal transmission to the botulinum neu- modulating the effect of a botulinum neurotoxin compo- rotoxin composition. The invention also relates to com- sition, that is accelerating the onset of action and /or ex- positions comprising at least one postsynaptic inhibitor tending the duration of action and/or enhancing the in- of cholinergic neuronal transmission and a botulinum tensity of action of a botulinum neurotoxin composition, neurotoxin, and their uses for treating aesthetic or ther- comprising adding at least one postsynaptic inhibitor of apeutic conditions. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Application Note 747014H HPLC Ana Llys Is of Th E M Ol Ecul Ar M Ass of L Ow-Mlmolecul Ar-Mhmass Hepari Ns Using the Method Described in the European Pharmacopoeia
Application Note 747014H HPLC ana llys is o f the Mo lecul ar M ass of L ow-MlMolecu lar-MHMass Hepar ins using the method described in the European Pharmacopoeia Introduction Heparins are a class of mucopolysaccharide obtained from the porcine small intestine and is widely used in the pharmaceutical field as an anticlotting drug for the treatment and prevention of thromboembolism, treatment of disseminated intravascular coagulation syndrome, and as an anti-blood clilotting agent when using extracorporeal bloo d ciliirculating apparatus such as bloo d dia lys isand artific ial heart/lung. Low-molecular-mass heparin is produced by enzymatic treatment and chemical manipulation of heparin and is used as an anticlotting drug similarly to heparin due to its more predictable pharmacokinetics. Low-molecular-mass heparin is classified as parnaparin, dalteparin, enoxaparin etc., by decomposition method and molecular weight distribution. In the European Pharmacopoeia 8th Edition (EP), the molecular weight measurement method is defined in the pharmaceutical products article of low-molecular-mass heparin, the average molecular weight and molecular weight distribution are also defined in the pharmaceutical article for low- molecular-mass heparin (sodium) and low-molecular-mass heparin (calcium) 1)-7). Size exclusion chromatography (SEC) combined with a UV-Visible and Refractive Index detection is defined as the measurement method. In the analytical method, a molecular weight calibration curve using low- molecular-mass heparin is created using low-molecular-mass heparin as the reference sample. In common molecular weight measurement methods using SEC, a calibration curve is created from the retention volume and molecular weight; this common method is used for oligomers and polymers where the molecular weight has already been determined and used as a standard sample. -

WHO Drug Information Vol. 04, No. 4, 1990
WHO DRUG INFORMATION VOLUME 4 • NUMBER 4 • 1990 PROPOSED INN LIST 64 INTERNATIONAL NONPROPRIETARY NAMES FOR PHARMACEUTICAL SUBSTANCES WORLD HEALTH ORGANIZATION • GENEVA WHO Drug Information WHO Drug Information provides an cerned with the rational use of overview of topics relating to drug drugs. In effect, the journal seeks development and regulation that to relate regulatory activity to are of current relevance and im therapeutic practice. It also aims to portance, and will include the lists provide an open forum for debate. of proposed and recommended In Invited contributions will portray a ternational Nonproprietary Names variety of viewpoints on matters of for Pharmaceutical Substances general policy with the aim of (INN). Its contents reflect, but do stimulating discussion not only in not present, WHO policies and ac these columns but wherever rel tivities and they embrace socio evant decisions on this subject economic as well as technical mat have to be taken. ters. WHO Drug Information is pub The objective is to bring issues that lished 4 times a year in English are of primary concern to drug and French. regulators and pharmaceutical manufacturers to the attention of a Annual subscription: Sw.fr. 50.— wide audience of health profes Airmail rate: Sw.fr. 60.— sionals and policy-makers con Price per copy: Sw.fr. 15.— © World Health Organization 1990 Authors alone are responsible for views expressed Publications of the World Health Organization in signed contributions. enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal The mention of specific companies or of certain Copyright Convention. For rights of reproduc manufacturers' products does not imply that they are tion or translation, in part or in toto, applica endorsed or recommended by the World Health tion should be made to: Chief, Office of Organization in preference to others of a similar na Publications, World Health Organization, ture which are not mentioned. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic