Endocrine Correlates of Social and Reproductive Behaviours in a Group
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Inventory of Avian Species in Aldesa Valley, Saudi Arabia
14 5 LIST OF SPECIES Check List 14 (5): 743–750 https://doi.org/10.15560/14.5.743 An inventory of avian species in Aldesa Valley, Saudi Arabia Abdulaziz S. Alatawi1, Florent Bled1, Jerrold L. Belant2 1 Mississippi State University, Forest and Wildlife Research Center, Carnivore Ecology Laboratory, Box 9690, Mississippi State, MS, USA 39762. 2 State University of New York, College of Environmental Science and Forestry, 1 Forestry Drive, Syracuse, NY, USA 13210. Corresponding author: Abdulaziz S. Alatawi, [email protected] Abstract Conducting species inventories is important to provide baseline information essential for management and conserva- tion. Aldesa Valley lies in the Tabuk Province of northwest Saudi Arabia and because of the presence of permanent water, is thought to contain high avian richness. We conducted an inventory of avian species in Aldesa Valley, using timed area-searches during May 10–August 10 in 2014 and 2015 to detect species occurrence. We detected 6860 birds belonging to 19 species. We also noted high human use of this area including agriculture and recreational activities. Maintaining species diversity is important in areas receiving anthropogenic pressures, and we encourage additional surveys to further identify species occurrence in Aldesa Valley. Key words Arabian Peninsula; bird inventory; desert fauna. Academic editor: Mansour Aliabadian | Received 21 April 2016 | Accepted 27 May 2018 | Published 14 September 2018 Citation: Alatawi AS, Bled F, Belant JL (2018) An inventory of avian species in Aldesa Valley, Saudi Arabia. Check List 14 (5): 743–750. https:// doi.org/10.15560/14.5.743 Introduction living therein (Balvanera et al. -

Percy Fitzpatrick Institute of African Ornithology Annual Report
Percy FitzPatrick Institute DST/NRF Centre of Excellence Annual Report January – December 2009 Department of Zoology University of Cape Town Private Bag X3 Rondebosch 7701 SOUTH AFRICA +27 (0)21 650 3290/1 [email protected] http://www.fitzpatrick.uct.ac.za University of Cape Town Contents Director’s report 1 Staff and Students 3 Research Programmes & Initiatives • Systematics and Biogeography 5 • Cooperation and Sociality in birds 13 • Rarity and Conservation of African birds 19 • Island Conservation 26 • Seabird Research 28 • Raptor Research 33 • Spatial Parasitology and Epidemiology 36 • Pattern-process Linkages in Landscape 39 Ecology • Environmental & Resource Economics 41 • Climate Change Vulnerability and 44 Adaptation • And a Miscellany 49 Conservation Biology Masters 53 Board Members: Programme Niven Library 55 Mr M. Anderson (BirdLife SA) Scientific Publications 59 Mr H. Amoore (UCT, Registrar) Dr G. Avery (Wildlife and Environment Society of Southern Africa) Semi-popular Publications 63 Prof. K. Driver (UCT, Dean of Science, Chairman) Prof. P.A.R. Hockey (UCT, Director, PFIAO) Seminars 2009 65 Assoc. Prof. J. Hoffmann (UCT, HoD, Zoology) Mr P.G. Johnson (co-opted) Dr J. McNamara (UCT, Development & Alumni Dept) Prof. M.E. Meadows (UCT, HoD, ENGEO) Mr C.A.F. Niven (FitzPatrick Memorial Trust) Mr J.D.F. Niven (FitzPatrick Memorial Trust) Mr P.N.F. Niven (FitzPatrick Memorial Trust) Mr F. van der Merwe (co-opted) Prof. D. Visser (UCT, Chairman, URC) The Annual Report may also be viewed on the Percy FitzPatrick Institute's website: http://www.fitzpatrick.uct.ac.za Director’s Report Director’s Report To say that 2009 was a busy and eventful year would be an understatement! Early in January, Doug Loewenthal, Graeme Oatley and I participated in the Biodiversity Academy at De Hoop Nature Reserve. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Recording Some of Breeding Birds in Mehmedan Region of Republic Yemen
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library European Journal of Experimental Biology, 2014, 4(1):625-632 ISSN: 2248 –9215 CODEN (USA): EJEBAU Recording some of breeding birds in Mehmedan region of Republic Yemen Fadhl Adullah Nasser Balem and Mohamed Saleh Alzokary Biology Department, Aden University, Yaman _____________________________________________________________________________________________ ABSTRACT Mehmedan region is always green and there are different trees, shrubs, herbs and a lot of land which cultivated by corn, millet and other monetary plants. The site has been identified by the authors as an important Bird Area and especially for passerines breeding birds. Aim of this paper is to recording of some breeding birds.Many field visits during the year (2012) were conducted and (13) breeding bird species were recoded, these birds relating to (5) Orders, (10) Families, and (11) Genera. Key words: Breeding birds, Mehmedan, Yemen. _____________________________________________________________________________________________ INTRODUCTION At present time about (432) bird species were recorded in avifauna of Yemen of which (1) is endemic, (2) have been introduced by humans, and (25) are rare or accidental, (14) species are globally threatened.Mehmedan region located in southern Tehama which defined as lying south of (21 0N) along the Saudi Arabian and Yemen Red Sea lowlands and east along the Gulf of Aden to approximately (46 0E).Temperatures and humidity greatly increase southwards and rainfall decreases but the area has many permanent water courses and much subsurface water due to the considerable rub-off of rainwater from the highlands. Consequently there is much more vegetation in the wadis and there is a good deal of traditional, small scale agriculture mostly of millet, sorghum and vegetables[1]. -

Cascading Ecological Effects from Local Extirpation of an Ecosystem Engineer in the Arava Desert
This is a repository copy of Cascading ecological effects from local extirpation of an ecosystem engineer in the Arava desert. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/132128/ Version: Accepted Version Article: Shanas, U, Gavish, Y orcid.org/0000-0002-6025-5668, Bernheim, M et al. (3 more authors) (2018) Cascading ecological effects from local extirpation of an ecosystem engineer in the Arava desert. Canadian Journal of Zoology, 96 (5). pp. 466-472. ISSN 0008-4301 https://doi.org/10.1139/cjz-2017-0114 Copyright remains with the author(s) or their institution(s). This is an author produced version of a paper published in the Canadian Journal of Zoology. Uploaded in accordance with the publisher's self-archiving policy. Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Cascading ecological effects from local extirpation of an ecosystem engineer in the Arava desert Uri Shanasa,b, Yoni Gavishc, Mai Bernheimb, Shacham Mittlerb, Yael Olekb, Alon Tald a Department of Biology and Environment, University of Haifa Oranim, Tivon 36006, Israel. -

A Checklist of Birds of Kerala, India
Journal of Threatened Taxa | www.threatenedtaxa.org | 17 November 2015 | 7(13): 7983–8009 A checklist of birds of Kerala, India Praveen J ISSN 0974-7907 (Online) B303, Shriram Spurthi, ITPL Main Road, Brookefields, Bengaluru, Karnataka 560037, India ISSN 0974-7893 (Print) Communication Short [email protected] OPEN ACCESS Abstract: A checklist of birds of Kerala State is presented in this pa- significant inventory of birds of Kerala was by Ferguson per. Accepted English names, scientific binomen, prevalent vernacular & Bourdillon (1903–04) who provided an annotated names in Malayalam, IUCN conservation status, endemism, Wildlife (Protection) Act schedules, and the appendices in the CITES, pertain- checklist of 332 birds from the princely state of ing to the birds of Kerala are also given. The State of Kerala has 500 Travancore. However, the landmark survey of the states species of birds, 17 of which are endemic to Western Ghats, and 24 species fall under the various threatened categories of IUCN. of Travancore and Cochin by Dr. Salim Ali in 1933–34 is widely accepted as the formal foundation in ornithology Keywords: CITES, endemism, Malayalam name, vernacular name, of Kerala. These surveys resulted in two highly popular Western Ghats, Wildlife (Protection) Act. books, The Birds of Travancore and Cochin (Ali 1953) and Birds of Kerala (Ali 1969); the latter listed 386 species. After two decades, Neelakantan et al. (1993) compiled Birds are one of the better studied groups of information on 95 bird species that were subsequently vertebrates in Kerala. The second half of 19th century recorded since Ali’s work. Birds of Kerala - Status and was dotted with pioneering contributions from T.C. -

Elena C. Berg
ELENA C. BERG Department of Computer Science, Mathematics & Environmental Science The American University of Paris 6, rue du Colonel Combes 75007 Paris France Email: [email protected] Web: http://www.aup.edu/profile/eberg http://www.ioe.ucla.edu/ctr/staff/Berg_Elena.html CURRENT/RECENT POSITIONS Associate Professor, Department of Computer Science, Mathematics & Environmental Science, The American University of Paris, France, August 2016 – Present Assistant Professor, Department of Computer Science, Mathematics & Environmental Science, The American University of Paris, France, January 2014 – July 2016 Senior Research Fellow, Center for Tropical Research, University of California, Los Angeles October 2006 – Present EDUCATION Ph.D., Animal Behavior, University of California, Davis. GPA: 4.0. June 2004. Fully funded through numerous grants, scholarships, and teaching assistantships Dissertation: Parentage, Kinship, and Group Structure in the White-throated Magpie-Jay (Calocitta formosa), a Cooperative Breeder with Female Helpers. Advisor: Dr. John Eadie Master of Philosophy, BiologiCal Anthropology, University of Cambridge, England. September 1996. Recipient of 1995 British Marshall Fellowship Thesis: Patterns of Rank-related Mating Success and Female Choice in Baboons and Macaques. Advisor: Dr. Phyllis Lee BaChelor of Arts, Anthropology anD College SCholar (interdisciplinary independent major), Cornell University, Ithaca, NY. GPA 3.9. May 1995. Junior Year Abroad, University of Hamburg, Hamburg, Germany. 1993-1994. TEACHING & RESEARCH INTERESTS -

Bird Diversity of Protected Areas in the Munnar Hills, Kerala, India
PRAVEEN & NAMEER: Munnar Hills, Kerala 1 Bird diversity of protected areas in the Munnar Hills, Kerala, India Praveen J. & Nameer P. O. Praveen J., & Nameer P.O., 2015. Bird diversity of protected areas in the Munnar Hills, Kerala, India. Indian BIRDS 10 (1): 1–12. Praveen J., B303, Shriram Spurthi, ITPL Main Road, Brookefields, Bengaluru 560037, Karnataka, India. Email: [email protected] Nameer P. O., Centre for Wildlife Studies, College of Forestry, Kerala Agricultural University, KAU (PO), Thrissur 680656, Kerala, India. India. [email protected] Introduction Table 1. Protected Areas (PA) of Munnar Hills The Western Ghats, one of the biodiversity hotspots of the Protected Area Abbreviation Area Year of world, is a 1,600 km long chain of mountain ranges running (in sq.km.) formation parallel to the western coast of the Indian peninsula. The region Anamudi Shola NP ASNP 7.5 2003 is rich in endemic fauna, including birds, and has been of great biogeographical interest. Birds have been monitored regularly Eravikulam NP ENP 97 1975 in the Western Ghats of Kerala since 1991, with more than 60 Kurinjimala WLS KWLS 32 2006 surveys having been carried out in the entire region (Praveen & Pampadum Shola NP PSNP 11.753 2003 Nameer 2009). This paper is a result of such a survey conducted in December 2012 supplemented by relevant prior work in this area. Anamalais sub-cluster in southern Western Ghats (Nair 1991; Das Munnar Hills (10.083°–10.333°N, 77.000°–77.617°E), et al. 2006). Anamudi (2685 m), the highest peak in peninsular forming part of the High Ranges of Western Ghats, also known as India, lies in these hills inside Eravikulam National Park (NP). -

AERC Wplist July 2015
AERC Western Palearctic list, July 2015 About the list: 1) The limits of the Western Palearctic region follow for convenience the limits defined in the “Birds of the Western Palearctic” (BWP) series (Oxford University Press). 2) The AERC WP list follows the systematics of Voous (1973; 1977a; 1977b) modified by the changes listed in the AERC TAC systematic recommendations published online on the AERC web site. For species not in Voous (a few introduced or accidental species) the default systematics is the IOC world bird list. 3) Only species either admitted into an "official" national list (for countries with a national avifaunistic commission or national rarities committee) or whose occurrence in the WP has been published in detail (description or photo and circumstances allowing review of the evidence, usually in a journal) have been admitted on the list. Category D species have not been admitted. 4) The information in the "remarks" column is by no mean exhaustive. It is aimed at providing some supporting information for the species whose status on the WP list is less well known than average. This is obviously a subjective criterion. Citation: Crochet P.-A., Joynt G. (2015). AERC list of Western Palearctic birds. July 2015 version. Available at http://www.aerc.eu/tac.html Families Voous sequence 2015 INTERNATIONAL ENGLISH NAME SCIENTIFIC NAME remarks changes since last edition ORDER STRUTHIONIFORMES OSTRICHES Family Struthionidae Ostrich Struthio camelus ORDER ANSERIFORMES DUCKS, GEESE, SWANS Family Anatidae Fulvous Whistling Duck Dendrocygna bicolor cat. A/D in Morocco (flock of 11-12 suggesting natural vagrancy, hence accepted here) Lesser Whistling Duck Dendrocygna javanica cat. -

A Checklist of the Birds of Goa, India
BAIDYA & BHAGAT: Goa checklist 1 A checklist of the birds of Goa, India Pronoy Baidya & Mandar Bhagat Baidya, P., & Bhagat, M., 2018. A checklist of the birds of Goa, India. Indian BIRDS 14 (1): 1–31. Pronoy Baidya, TB-03, Center for Ecological Sciences, Indian Institute of Science, Bengaluru 560012, Karnataka, India. And, Foundation for Environment Research and Conservation, C/o 407, III-A, Susheela Seawinds, Alto-Vaddem, Vasco-da-Gama 403802, Goa, India. E-mail: [email protected] [Corresponding author] [PB] Mandar Bhagat, ‘Madhumangal’, New Vaddem,Vasco-da-Gama 403802, Goa, India. E-mail: [email protected] [MB] Manuscript received on 15 November 2017. We dedicate this paper to Heinz Lainer, for his commitment to Goa’s Ornithology. Abstract An updated checklist of the birds of Goa, India, is presented below based upon a collation of supporting information from museum specimens, photographs, audio recordings of calls, and sight records with sufficient field notes. Goa has 473 species of birds of which 11 are endemic to the Western Ghats, 19 fall under various categories of the IUCN Red List of Threatened Species, and 48 are listed in Schedule I Part (III) of The Indian Wild Life (Protection) Act, 1972. 451 species have been accepted into the checklist based on specimens in various museums or on photographs, while 22 have been accepted based on sight record. A secondary list of unconfirmed records is also discussed in detail. Introduction that is about 125 km long. The southern portion of these ghats, Goa, India’s smallest state, sandwiched between the Arabian within Goa, juts out towards the Arabian Sea, at Cabo de Rama, Sea in the west and the Western Ghats in the east, is home to and then curves inland. -

The Birds of the Highlands of South-West Saudi Arabia and Adjacent Parts of the Tihama: July 2010 (Abba Survey 42)
THE BIRDS OF THE HIGHLANDS OF SOUTH-WEST SAUDI ARABIA AND ADJACENT PARTS OF THE TIHAMA: JULY 2010 (ABBA SURVEY 42) by Michael C. Jennings, Amar R. H. Al-Momen and Jabr S. Y. Haresi December 2010 THE BIRDS OF THE HIGHLANDS OF SOUTH-WEST SAUDI ARABIA AND ADJACENT PARTS OF THE TIHAMA: JULY 2010 (ABBA SURVEY 42) by Michael C. Jennings1, Amar R. H. Al-Momen2 and Jabr S. Y. Haresi2 December 2010 SUMMARY The objective of the survey was to compare habitats and bird life in the Asir region, particularly Jebal Souda and the Raydah escarpment protected area of the Saudi Wildlife Commission, and adjacent regions of the tihama, with those observed in July 1987 (Jennings, et al., 1988). The two surveys were approximately the same length and equal amounts of time were spent in the highlands and on the tihama. A number of walked censuses were carried out during 2010 on Jebal Souda, using the same methodology as walked censuses in 1987, and the results are compared. Broadly speaking the comparison of censuses revealed that in 2010 there were less birds and reduced diversity on the Jebal Souda plateau, compared to 1987. However in the Raydah reserve the estimates of breeding bird populations compiled in the mid 1990s was little changed as far as could be assessed in 2010. The highland region of south-west Saudi Arabia, especially Jebal Souda, has been much developed since the 1987 survey and is now an important internal recreation and resort area. This has lead to a reduction in the region’s importance for terraced agriculture. -
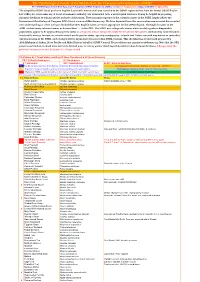
Simplified-ORL-2019-5.1-Final.Pdf
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part F: Simplified OSME Region List (SORL) version 5.1 August 2019. (Aligns with ORL 5.1 July 2019) The simplified OSME list of preferred English & scientific names of all taxa recorded in the OSME region derives from the formal OSME Region List (ORL); see www.osme.org. It is not a taxonomic authority, but is intended to be a useful quick reference. It may be helpful in preparing informal checklists or writing articles on birds of the region. The taxonomic sequence & the scientific names in the SORL largely follow the International Ornithological Congress (IOC) List at www.worldbirdnames.org. We have departed from this source when new research has revealed new understanding or when we have decided that other English names are more appropriate for the OSME Region. The English names in the SORL include many informal names as denoted thus '…' in the ORL. The SORL uses subspecific names where useful; eg where diagnosable populations appear to be approaching species status or are species whose subspecies might be elevated to full species (indicated by round brackets in scientific names); for now, we remain neutral on the precise status - species or subspecies - of such taxa. Future research may amend or contradict our presentation of the SORL; such changes will be incorporated in succeeding SORL versions. This checklist was devised and prepared by AbdulRahman al Sirhan, Steve Preddy and Mike Blair on behalf of OSME Council. Please address any queries to [email protected].