In Some Guans (Cracidae) of the Andes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
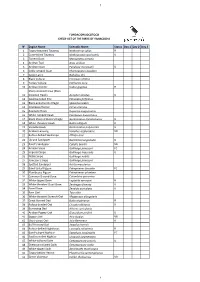
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

List of the Birds of Peru Lista De Las Aves Del Perú
LIST OF THE BIRDS OF PERU LISTA DE LAS AVES DEL PERÚ By/por MANUEL A. -

Neotropical News Neotropical News
COTINGA 1 Neotropical News Neotropical News Brazilian Merganser in Argentina: If the survey’s results reflect the true going, going … status of Mergus octosetaceus in Argentina then there is grave cause for concern — local An expedition (Pato Serrucho ’93) aimed extinction, as in neighbouring Paraguay, at discovering the current status of the seems inevitable. Brazilian Merganser Mergus octosetaceus in Misiones Province, northern Argentina, During the expedition a number of sub has just returned to the U.K. Mergus tropical forest sites were surveyed for birds octosetaceus is one of the world’s rarest — other threatened species recorded during species of wildfowl, with a population now this period included: Black-fronted Piping- estimated to be less than 250 individuals guan Pipile jacutinga, Vinaceous Amazon occurring in just three populations, one in Amazona vinacea, Helmeted Woodpecker northern Argentina, the other two in south- Dryocopus galeatus, White-bearded central Brazil. Antshrike Biata s nigropectus, and São Paulo Tyrannulet Phylloscartes paulistus. Three conservation biologists from the U.K. and three South American counter PHIL BENSTEAD parts surveyed c.450 km of white-water riv Beaver House, Norwich Road, Reepham, ers and streams using an inflatable boat. Norwich, NR10 4JN, U.K. Despite exhaustive searching only one bird was located in an area peripheral to the species’s historical stronghold. Former core Black-breasted Puffleg found: extant areas (and incidently those with the most but seriously threatened. protection) for this species appear to have been adversely affected by the the Urugua- The Black-breasted Puffleg Eriocnemis í dam, which in 1989 flooded c.80 km of the nigrivestis has been recorded from just two Río Urugua-í. -

Bird) Species List
Aves (Bird) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Archosauria, Aves Order (O:) and Family (F:) English Name2 Scientific Name3 O: Tinamiformes (Tinamous) F: Tinamidae (Tinamous) Great Tinamou Tinamus major Highland Tinamou Nothocercus bonapartei O: Galliformes (Turkeys, Pheasants & Quail) F: Cracidae Black Guan Chamaepetes unicolor (Chachalacas, Guans & Curassows) Gray-headed Chachalaca Ortalis cinereiceps F: Odontophoridae (New World Quail) Black-breasted Wood-quail Odontophorus leucolaemus Buffy-crowned Wood-Partridge Dendrortyx leucophrys Marbled Wood-Quail Odontophorus gujanensis Spotted Wood-Quail Odontophorus guttatus O: Suliformes (Cormorants) F: Fregatidae (Frigatebirds) Magnificent Frigatebird Fregata magnificens O: Pelecaniformes (Pelicans, Tropicbirds & Allies) F: Ardeidae (Herons, Egrets & Bitterns) Cattle Egret Bubulcus ibis O: Charadriiformes (Sandpipers & Allies) F: Scolopacidae (Sandpipers) Spotted Sandpiper Actitis macularius O: Gruiformes (Cranes & Allies) F: Rallidae (Rails) Gray-Cowled Wood-Rail Aramides cajaneus O: Accipitriformes (Diurnal Birds of Prey) F: Cathartidae (Vultures & Condors) Black Vulture Coragyps atratus Turkey Vulture Cathartes aura F: Pandionidae (Osprey) Osprey Pandion haliaetus F: Accipitridae (Hawks, Eagles & Kites) Barred Hawk Morphnarchus princeps Broad-winged Hawk Buteo platypterus Double-toothed Kite Harpagus bidentatus Gray-headed Kite Leptodon cayanensis Northern Harrier Circus cyaneus Ornate Hawk-Eagle Spizaetus ornatus Red-tailed -

Creación De Un Manual Interpretativo Para El Buen
CARRERA ADMINISTRACIÓN TURÍSTICA Y HOTELERA CREACIÓN DE UN MANUAL INTERPRETATIVO SOBRE EL BUEN AVISTAMIENTO DE AVES EN LA RESERVA YANACOCHA UBICADA EN LA PARROQUIA DE NONO CANTÓN QUITO PROVINCIA PICHINCHA CON EL PROPÓSITO DE DAR A CONOCER EL AVITURISMO Proyecto de investigación previo a la obtención de título de tecnólogo en Administración Turística y Hotelera Autora: Amanda Estefania Tituaña Espinosa Tutor: Ing. Ximena Almeida Quito, Diciembre 2018 i Declaratoria Declaro que la investigación es absolutamente original, autentica, personal, que se han citado las fuentes correspondientes y en su ejecución se respetaron las disposiciones legales que protegen los derechos de autor vigentes. Las ideas, doctrinas resultados y conclusiones a los que he llegado son de mi absoluta responsabilidad. Amanda Estefania Tituaña Espinosa CC 1750804161 CREACIÓN DE UN MANUAL INTERPRETATIVO SOBRE EL BUEN AVISTAMIENTO DE AVES EN LA RESERVA YANACOCHA UBICADA EN LA PARROQUIA DE NONO CANTON QUITO PROVINCIA PICHINCHA CON EL PROPOSITO DE DAR A CONOCER EL AVITURISMO ii Licencia De Uso No Comercial Yo, Amanda Estefania Tituaña Espinosa portadora de la cedula de ciudadanía asignada Con el No. 175080416-1 de conformidad con lo establecido en el Artículo 110 del Código de Economía Social de los Conocimientos, la Creación y la Innovación (INGENIOS) que dice: “En el caso de las obras creadas en centros educativos,universidades,escuelas politécnicas, institutos superiores tecnicos,tecnólogos, pedagógicos, de arte y los conservatorios superiores , e institutos públicos de investigación como resultado de su actividad académica o de investigación tales como trabajos de titulación, proyectos de investigación o innovación, articulo académico , u otros análogos , sin perjuicio de que pueda existir relación de dependencia , la titularidad de los derechos patrimoniales corresponderá a los autores . -

Neotropical Birding 24 2 Neotropical Species ‘Uplisted’ to a Higher Category of Threat in the 2018 IUCN Red List Update
>> FEATURE RED LIST 2018 The 2018 IUCN Red List in the Neotropics James Lowen, Hannah Wheatley, Claudia Hermes, Ian Burfield and David Wege Neotropical Birding 21 featured a summary of the key implications for the Neotropics of the 2016 IUCN Red List for birds. This article briefs readers on the main changes from the 2018 update. s part of its role as the IUCN Red List BirdLife’s Red List team updated the Authority for birds, BirdLife International information available for roughly 2,300 species A is responsible for assessing the global worldwide. Globally, this resulted in changes to conservation status of each of the world’s 11,000 the categorisation of 89 species; 58 species were or so bird species, allocating each to a category ‘uplisted’ to a higher category of threat, whilst ranging from Least Concern to Extinct. The latest roughly half that number – 31 species – were update was published in November 2018 (BirdLife ‘downlisted’. In the Neotropics, 13 species were International 2018). Although much more modest uplisted (Fig. 2) and slightly more – 18 – were in reach than the comprehensive update carried downlisted (Fig. 5). Now let’s take a closer look at out in 2016, whose Neotropical dimension was the individual changes, largely using information discussed in Symes et al. (2017), the 2018 revamp made available on BirdLife’s ‘Globally Threatened contains a suite of interesting changes for species Bird Forums’ (8 globally-threatened-bird- occurring in the Neotropical Bird Club region that forums.birdlife.org). Is the picture quite as rosy as are worth drawing to readers’ collective attention. -

RSG Book Template 2011 V4 051211
IUCN IUCN, International Union for Conservation of Nature, helps the world find pragmatic solutions to our most pressing environment and development challenges. IUCN works on biodiversity, climate change, energy, human livelihoods and greening the world economy by supporting scientific research, managing field projects all over the world, and bringing governments, NGOs, the UN and companies together to develop policy, laws and best practice. IUCN is the world’s oldest and largest global environmental organization, with more than 1,200 government and NGO members and almost 11,000 volunteer experts in some 160 countries. IUCN’s work is supported by over 1,000 staff in 60 offices and hundreds of partners in public, NGO and private sectors around the world. IUCN Species Survival Commission (SSC) The SSC is a science-based network of close to 8,000 volunteer experts from almost every country of the world, all working together towards achieving the vision of, “A world that values and conserves present levels of biodiversity.” Environment Agency - ABU DHABI (EAD) The EAD was established in 1996 to preserve Abu Dhabi’s natural heritage, protect our future, and raise awareness about environmental issues. EAD is Abu Dhabi’s environmental regulator and advises the government on environmental policy. It works to create sustainable communities, and protect and conserve wildlife and natural resources. EAD also works to ensure integrated and sustainable water resources management, and to ensure clean air and minimize climate change and its impacts. Denver Zoological Foundation (DZF) The DZF is a non-profit organization whose mission is to “secure a better world for animals through human understanding.” DZF oversees Denver Zoo and conducts conservation education and biological conservation programs at the zoo, in the greater Denver area, and worldwide. -

Standards for Ground Feeding Bird Sanctuaries
Global Federation of Animal Sanctuaries Standards For Ground Feeding Bird Sanctuaries Version: June 2013 ©2012 Global Federation of Animal Sanctuaries i Global Federation of Animal Sanctuaries – Standards for Ground Feeding Bird Sanctuaries Table of Contents INTRODUCTION 1 GFAS PRINCIPLES 1 ANIMALS COVERED BY THESE STANDARDS 1 STANDARDS UPDATES 2 GROUND FEEDING BIRD STANDARDS 3 GROUND FEEDING BIRD HOUSING 3 H-1. Types of Space and Size 3 H-2. Containment 5 H-3. Ground and Plantings 6 H-4. Gates and Doors 7 H-5. Shelter 8 H-6. Enclosure Furniture 8 H-7. Sanitation 9 H-8. Temperature, Humidity, Ventilation, Lighting 11 PHYSICAL FACILITIES AND ADMINISTRATION 12 PF-1. Overall Safety of Facilities 12 PF-2. Water Drainage and Testing 13 PF-3. Life Support 13 PF-4. Hazardous Materials Handling 13 PF-5. Security: Avian Enclosures 14 PF-6. Perimeter Boundary and Inspections, and Maintenance 14 PF-7. Security: General Safety Monitoring 15 PF-8. Insect and Rodent Control 15 PF-9. Record Keeping 16 PF-10. Animal Transport 16 NUTRITION REQUIREMENTS 18 N-1. Water 18 N-2. Diet 18 N-3. Food Presentation and Feeding Techniques 20 N-4. Food Storage 21 N-5. Food Handling 21 VETERINARY CARE 22 V-1. General Medical Program and Staffing 22 V-2. On-Site and Off-Site Veterinary Facilities 22 V-3. Preventative Medicine Program 23 V-4. Diagnostic Services, Surgical, Treatment and Necropsy Facilities 23 V-5. Quarantine and Isolation of Ground Feeding Birds 25 V-6. Medical Records and Controlled Substances 26 i Global Federation of Animal Sanctuaries – Standards for Ground Feeding Bird Sanctuaries V-7. -

Assemblages of Bird and Mammal Communities in Two Major Ecological Units of the Andean Highland Plateau of Southern Peru
Ecología Aplicada, 6(1,2), 2007 Presentado: 05/07/2007 ISSN 1726-2216 Aceptado: 08/12/2007 Depósito legal 2002-5474 © Departamento Académico de Biología, Universidad Nacional Agraria La Molina, Lima – Perú. ASSEMBLAGES OF BIRD AND MAMMAL COMMUNITIES IN TWO MAJOR ECOLOGICAL UNITS OF THE ANDEAN HIGHLAND PLATEAU OF SOUTHERN PERU ESTRUCTURA DE LAS COMUNIDADES DE AVES Y MAMÍFEROS EN DOS UNIDADES ECOLÓGICAS DE LOS ANDES DEL SUR DEL PERÚ Oswaldo Ramirez1, Margarita Arana1, Enrique Bazán1, Angel Ramirez2 y Asunción Cano2 Abstract Grasslands in the Andean highlands plateau of southern Peru have been considered as a single and homogeneous dry habitat also known as Puna. However, in some regions, a highest rainfall regimen is found, and the name of wet puna has begun to be used by some authors. Since no studies have been carrying out specifically to test if dry and wet puna are different ecological units, we chose two representative localities of each one of these habitat to evaluate assemblages of bird and mammal communities and their continuity or independence between these apparently similar habitats. Our results suggest that wet puna has different mammal diversity than dry puna, and a heterogeneous bird community with species that have been previously reported exclusively in paramo or exclusively in puna. In spite of the apparent uniform flora in the Andean highlands of South Peru, data suggest that rainfall regimens produce a mosaic of habitats that will be determining ecological barriers for terrestrial mammals, in particular for small mammals. Key words: Andean grasslands, Andean plateau, Cuzco, Oriental Cordillera, paramo, puna, Puno. Resumen Los Pajonales de los Andes del sur del Perú, también conocidos como Puna, son considerados como hábitats homogéneos y secos. -

Ecuador Birding the Chocó-Andes Region: Western and Eastern Slopes of Ecuador November 25-December 4, 2018
MINDO CLOUD FOREST ECUADOR Birding the Chocó-Andes Region: Western and Eastern Slopes of Ecuador NOVEMBER 25-DECEMBER 4, 2018 Per square mile, Ecuador has the PROGRAM HIGHLIGHTS highest biodiversity in the world, including some 1,640 species of birds, • Explore diverse habitats at varying elevations at national and private reserves, including Alambi Cloud Forest Reserve, many of which are rare and endemic. the subtropical rainforest at Milpe Bird Sanctuary, páramo Discover the amazing contrasts of ecosystem at Antisana Ecological Reserve, and more. cloud forest, the Amazon, and Andean landscapes on this 10-day birding • Seek out some of Ecuador’s approximately 130 adventure. With assistance from an hummingbird species, including the Giant Hummingbird, Black-tailed Trainbearer, Sword-billed Hummingbird, expert guide, encounter a variety of Tourmaline Sunangel, and Glowing Puffleg. birds, plants, and other wildlife while traversing a selection of Ecuador’s • Identify other target species like the Andean Condor, 50 different ecosystems. The program Andean Cock-of-the-rock, Long-wattled Umbrellabird, Chocó Trogon, and dozens of tanagers. begins with a journey to Mindo Loma Private Reserve and its 17 acres of primary and secondary recovering cloud forest. Also experience the WHAt’s INCLUDED? Milpe Bird Sanctuary in the Chocó • Bilingual local guide Andean foothills, considered one of • Driver the finest sites in all of Ecuador. Along • Accommodations the way, meet a famous Ecuadorian • Activities ornithologist, view the snow-capped • Private transportation Antisana Volcano, and straddle the • Meals equator at the Middle of the World • Beverages with meals Monument. • Carbon offsetting BUFF-TAILED CORONET BY JULIAN LONDONO holbrooktravel.com | 800-451-7111 ANTISANA VOLCANO BY MARCIO RAMALHO ITINERARY areas of original cloud forest habitat and wildlife, where you will be able to experience the natural beauty and BLD = BREAKFAST, LUNCH, DINNER wonder of this very unique and biologically diverse environment. -

PERU: Manu and Machu Picchu Aug-Sept
Tropical Birding Trip Report PERU: Manu and Machu Picchu Aug-Sept. 2015 A Tropical Birding SET DEPARTURE tour PERU: MANU and MACHU PICCHU th th 29 August – 16 September 2015 Tour Leader: Jose Illanes Andean Cock-of-the-rock near Cock-of-the-rock Lodge! Species highlighted in RED are the ones illustrated with photos in this report. INTRODUCTION Not everyone is fortunate enough to visit Peru; a marvelous country that boasts a huge country bird list, which is second only to Colombia. Unlike our usual set departure, we started out with a daylong extension to Lomas de Lachay first, before starting out on the usual itinerary for the main tour. On this extra day we managed to 1 www.tropicalbirding.com +1-409-515-0514 [email protected] Page Tropical Birding Trip Report PERU: Manu and Machu Picchu Aug-Sept. 2015 find many extra birds like Peruvian Thick-knee, Least Seedsnipe, Peruvian Sheartail, Raimondi’s Yellow- Finch and the localized Cactus Canastero. The first site of the main tour was Huacarpay Lake, near the beautiful Andean city of Cusco (accessed after a short flight from Lima). This gave us a few endemic species like Bearded Mountaineer and Rusty-fronted Canastero; along with other less local species like Many-colored Rush-tyrant, Plumbeous Rail, Puna Teal, Andean Negrito and Puna Ibis. The following day we birded along the road towards Manu where we picked up birds like Peruvian Sierra-Finch, Chestnut-breasted Mountain-Finch, Spot-winged Pigeon, and a beautiful Peruvian endemic in the form of Creamy-crested Spinetail. We also saw Yungas Pygmy-Owl, Black-faced Ibis, Hooded and Scarlet-bellied Mountain- Tanagers, Red-crested Cotinga and the gorgeous Grass-green Tanager. -

Peru: Manu National Park with Machu Picchu Extension October 10-28, 2018 with Woody Wheeler
Peru: Manu National Park with Machu Picchu Extension October 10-28, 2018 with Woody Wheeler Guides: Andrea Molina and Woody Wheeler with Bill, David, Denise, John, Jerry, Judy, Karen, Linda, Lori, Mark, Pat and Robin Photo taken by Luke, a volunteer at Manu Learning Center Day 1: Lima to Pantanos de Villa, Pucusana, San Pedro Beach On a cool, misty morning we headed south from Lima along its extensive public beaches to the protected wetlands of Pantanos De Villa. As soon as we stepped of the bus onto the spongy, soft straw trails of the refuge, we were surrounded by birds. West Peruvian Doves with their enchanting blue eyes perched on the tops of shrubs. A Harris’ Hawk ousted a Black Vulture from one of the tall Naturalist Journeys, LLC PO Box 16545 Portal, AZ 85632 PH: 520.558.1146 Toll free 866.900.1146 Fax 650.471.7667 www.naturalistjourneys.com Email [email protected] Peru: Manu National Park with Machu Picchu Extension October 10-28, 2018 with Woody Wheeler perches, and a Long-tailed Mockingbird chose a utility wire for its perch. Competition for perching spots was fierce! Then came the next wave of birds as we ambled along the soft straw trails of the refuge: Yellow- bellied Seedeater, Lesser Goldfinch, Snowy and Great Egrets, as well as the appropriately named Many- colored Rush Tyrant. Andrea helped attract a Wren-like Rushbird by imitating its “clickety” call. In one of the refuge’s large ponds, we had good looks at majestic Great Grebe, Slate-colored Coot, and White-cheeked Pintail.