Seasonality and Elevational Migration in an Andean Bird Community
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Basilinna Genus (Aves: Trochilidae): an Evaluation Based on Molecular Evidence and Implications for the Genus Hylocharis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Revista Mexicana de Biodiversidad 85: 797-807, 2014 DOI: 10.7550/rmb.35769 The Basilinna genus (Aves: Trochilidae): an evaluation based on molecular evidence and implications for the genus Hylocharis El género Basilinna (Aves: Trochilidae): una evaluación basada en evidencia molecular e implicaciones para el género Hylocharis Blanca Estela Hernández-Baños1 , Luz Estela Zamudio-Beltrán1, Luis Enrique Eguiarte-Fruns2, John Klicka3 and Jaime García-Moreno4 1Museo de Zoología, Departamento de Biología Evolutiva, Facultad de Ciencias, Universidad Nacional Autónoma de México. Apartado postal 70- 399, 04510 México, D. F., Mexico. 2Departamento de Ecología Evolutiva, Instituto de Ecología, Universidad Nacional Autónoma de México. Apartado postal 70-275, 04510 México, D. F., Mexico. 3Burke Museum of Natural History and Culture, University of Washington, Box 353010, Seattle, WA, USA. 4Amphibian Survival Alliance, PO Box 20164, 1000 HD Amsterdam, The Netherlands. [email protected] Abstract. Hummingbirds are one of the most diverse families of birds and the phylogenetic relationships within the group have recently begun to be studied with molecular data. Most of these studies have focused on the higher level classification within the family, and now it is necessary to study the relationships between and within genera using a similar approach. Here, we investigated the taxonomic status of the genus Hylocharis, a member of the Emeralds complex, whose relationships with other genera are unclear; we also investigated the existence of the Basilinna genus. We obtained sequences of mitochondrial (ND2: 537 bp) and nuclear genes (AK-5 intron: 535 bp, and c-mos: 572 bp) for 6 of the 8 currently recognized species and outgroups. -

Bird Ecology, Conservation, and Community Responses
BIRD ECOLOGY, CONSERVATION, AND COMMUNITY RESPONSES TO LOGGING IN THE NORTHERN PERUVIAN AMAZON by NICO SUZANNE DAUPHINÉ (Under the Direction of Robert J. Cooper) ABSTRACT Understanding the responses of wildlife communities to logging and other human impacts in tropical forests is critical to the conservation of global biodiversity. I examined understory forest bird community responses to different intensities of non-mechanized commercial logging in two areas of the northern Peruvian Amazon: white-sand forest in the Allpahuayo-Mishana Reserve, and humid tropical forest in the Cordillera de Colán. I quantified vegetation structure using a modified circular plot method. I sampled birds using mist nets at a total of 21 lowland forest stands, comparing birds in logged forests 1, 5, and 9 years postharvest with those in unlogged forests using a sample effort of 4439 net-hours. I assumed not all species were detected and used sampling data to generate estimates of bird species richness and local extinction and turnover probabilities. During the course of fieldwork, I also made a preliminary inventory of birds in the northwest Cordillera de Colán and incidental observations of new nest and distributional records as well as threats and conservation measures for birds in the region. In both study areas, canopy cover was significantly higher in unlogged forest stands compared to logged forest stands. In Allpahuayo-Mishana, estimated bird species richness was highest in unlogged forest and lowest in forest regenerating 1-2 years post-logging. An estimated 24-80% of bird species in unlogged forest were absent from logged forest stands between 1 and 10 years postharvest. -

Partial Altitudinal Migration of the Near Threatened Satyr Tragopan Tragopan Satyra in the Bhutan Himalayas: Implications for Conservation in Mountainous Environments
Partial altitudinal migration of the Near Threatened satyr tragopan Tragopan satyra in the Bhutan Himalayas: implications for conservation in mountainous environments N AWANG N ORBU,UGYEN,MARTIN C. WIKELSKI and D AVID S. WILCOVE Abstract Relative to long-distance migrants, altitudinal mi- To view supplementary material for this article, please visit grants have been understudied, perhaps because of a percep- http://dx.doi.org/./S tion that their migrations are less complex and therefore easier to protect. Nonetheless, altitudinal migrants may be at risk as they are subject to ongoing anthropogenic pressure from land use and climate change. We used global position- Introduction ing system/accelerometer telemetry to track the partial alti- tudinal migration of the satyr tragopan Tragopan satyra in elative to long-distance migrants, altitudinal migrants central Bhutan. The birds displayed a surprising diversity of Rhave been understudied, perhaps because of a percep- migratory strategies: some individuals did not migrate, tion that their migrations are less complex and easier to pro- others crossed multiple mountains to their winter ranges, tect. In montane regions many species migrate altitudinally others descended particular mountains, and others as- up and down mountain slopes (Stiles, ; Powell & Bjork, cended higher up into the mountains in winter. In all ; Burgess & Mlingwa, ; Chaves-Champos et al., cases migration between summer breeding and winter ; Faaborg et al., ). Although attempts have been non-breeding grounds was accomplished largely by walk- made (Laymon, ; Cade & Hoffman, ; Powell & ing, not by flying. Females migrated in a south-easterly dir- Bjork, ; Chaves-Champos et al., ; Hess et al., ection whereas males migrated in random directions. -

TAG Operational Structure
PARROT TAXON ADVISORY GROUP (TAG) Regional Collection Plan 5th Edition 2020-2025 Sustainability of Parrot Populations in AZA Facilities ...................................................................... 1 Mission/Objectives/Strategies......................................................................................................... 2 TAG Operational Structure .............................................................................................................. 3 Steering Committee .................................................................................................................... 3 TAG Advisors ............................................................................................................................... 4 SSP Coordinators ......................................................................................................................... 5 Hot Topics: TAG Recommendations ................................................................................................ 8 Parrots as Ambassador Animals .................................................................................................. 9 Interactive Aviaries Housing Psittaciformes .............................................................................. 10 Private Aviculture ...................................................................................................................... 13 Communication ........................................................................................................................ -

Bird List Column A: 1 = 70-90% Chance Column B: 2 = 30-70% Chance Column C: 3 = 10-30% Chance
Colombia: Chocó Prospective Bird List Column A: 1 = 70-90% chance Column B: 2 = 30-70% chance Column C: 3 = 10-30% chance A B C Tawny-breasted Tinamou 2 Nothocercus julius Highland Tinamou 3 Nothocercus bonapartei Great Tinamou 2 Tinamus major Berlepsch's Tinamou 3 Crypturellus berlepschi Little Tinamou 1 Crypturellus soui Choco Tinamou 3 Crypturellus kerriae Horned Screamer 2 Anhima cornuta Black-bellied Whistling-Duck 1 Dendrocygna autumnalis Fulvous Whistling-Duck 1 Dendrocygna bicolor Comb Duck 3 Sarkidiornis melanotos Muscovy Duck 3 Cairina moschata Torrent Duck 3 Merganetta armata Blue-winged Teal 3 Spatula discors Cinnamon Teal 2 Spatula cyanoptera Masked Duck 3 Nomonyx dominicus Gray-headed Chachalaca 1 Ortalis cinereiceps Colombian Chachalaca 1 Ortalis columbiana Baudo Guan 2 Penelope ortoni Crested Guan 3 Penelope purpurascens Cauca Guan 2 Penelope perspicax Wattled Guan 2 Aburria aburri Sickle-winged Guan 1 Chamaepetes goudotii Great Curassow 3 Crax rubra Tawny-faced Quail 3 Rhynchortyx cinctus Crested Bobwhite 2 Colinus cristatus Rufous-fronted Wood-Quail 2 Odontophorus erythrops Chestnut Wood-Quail 1 Odontophorus hyperythrus Least Grebe 2 Tachybaptus dominicus Pied-billed Grebe 1 Podilymbus podiceps Magnificent Frigatebird 1 Fregata magnificens Brown Booby 2 Sula leucogaster ________________________________________________________________________________________________________ WINGS ● 1643 N. Alvernon Way Ste. 109 ● Tucson ● AZ ● 85712 ● www.wingsbirds.com (866) 547 9868 Toll free US + Canada ● Tel (520) 320-9868 ● Fax (520) -

New Distributional Bird Records from the Eastern Andean Slopes of Ecuador Istributio D
ISSN 1809-127X (online edition) © 2010 Check List and Authors Chec List Open Access | Freely available at www.checklist.org.br Journal of species lists and distribution N New distributional bird records from the eastern Andean slopes of Ecuador ISTRIBUTIO D 1,2,3* 4 RAPHIC G Alejandro Solano-Ugalde and Galo J. Real-Jibaja EO 1 G N O Fundación Imaymana, Paltapamba 476 San Pedro del Valle, Nayón. Quito, Ecuador. 2 Neblina Forest Natural History and Birding Tours, South America, Isla Floreana e8-129. Quito, Ecuador. 3 Natural History of Ecuador’s [email protected] Avifauna Group, 721 Foch y Amazonas. Quito, Ecuador. OTES 4 Real Nature, Travel Company, Casa Upano. Macas, Morona Santiago, Ecuador. N * Corresponding author. E-mail: Abstract: Distribution of birds is dynamic. Understanding, documentation and appropriate use of new records are essential, especially when managing threatened species. Here we present novel data regarding new distributional records for 17 bird species along the Amazonian slopes of the eastern Ecuadorian Andes. The new records fill gaps on our knowledge in the distribution, both in latitude and altitude. Although knowledge on the distribution of birds on mainland Ecuador has been well studied (Fjeldså Rostrhamus sociabilis ACCIPITRIDAE during recent years an inspiring number of articles have Snail Kite Cassin, 1854 - Two beenand Krabbe published 1990; documenting Ridgely and newGreenfield discoveries 2001; on2006), the individuals in juvenile plumage were seen flying over the distribution of birds in mainland Ecuador (e.g. Freile old-Zamora Airstrip on 6 March 2008, Zamora-Chinchipe et al. province (950 m a.s.l., 03°59’ S, 78°53’ W). -

Global Variation in Woodpecker Species Richness Shaped by Tree
Journal of Biogeography (J. Biogeogr.) (2017) 44, 1824–1835 ORIGINAL Global variation in woodpecker species ARTICLE richness shaped by tree availability † † Sigrid Kistrup Ilsøe1, , W. Daniel Kissling2, ,* , Jon Fjeldsa3, Brody Sandel4 and Jens-Christian Svenning1 1Section for Ecoinformatics and Biodiversity, ABSTRACT Department of Bioscience, Aarhus University, Aim Species richness patterns are generally thought to be determined by abi- DK-8000 Aarhus C, Denmark, 2Institute for otic variables at broad spatial scales, with biotic factors being only important Biodiversity and Ecosystem Dynamics (IBED), University of Amsterdam, 1090 GE at fine spatial scales. However, many organism groups depend intimately on Amsterdam, The Netherlands, 3Center for other organisms, raising questions about this generalization. As an example, Macroecology, Evolution and Climate at woodpeckers (Picidae) are closely associated with trees and woody habitats Natural History Museum of Denmark, because of multiple morphological and ecological specializations. In this study, University of Copenhagen, DK-1350 we test whether this strong biotic association causes woodpecker diversity to be Copenhagen K, Denmark, 4Department of closely linked to tree availability at a global scale. Biology, Santa Clara University, Santa Clara, Location Global. CA 95057, USA Methods We used spatial and non-spatial regressions to test for relationships between broad-scale woodpecker species richness and predictor variables describing current and deep-time availability of trees, current climate, Quater- nary climate change, human impact, topographical heterogeneity and biogeo- graphical region. We further used structural equation models to test for direct and indirect effects of predictor variables. Results There was a strong positive relationship between woodpecker species richness and current tree cover and annual precipitation, respectively. -
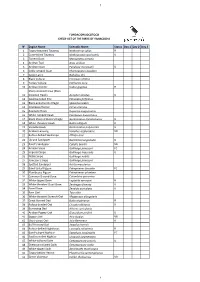
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

TOUR REPORT Southwestern Amazonia 2017 Final
For the first time on a Birdquest tour, the Holy Grail from the Brazilian Amazon, Rondonia Bushbird – male (Eduardo Patrial) BRAZIL’S SOUTHWESTERN AMAZONIA 7 / 11 - 24 JUNE 2017 LEADER: EDUARDO PATRIAL What an impressive and rewarding tour it was this inaugural Brazil’s Southwestern Amazonia. Sixteen days of fine Amazonian birding, exploring some of the most fascinating forests and campina habitats in three different Brazilian states: Rondonia, Amazonas and Acre. We recorded over five hundred species (536) with the exquisite taste of specialties from the Rondonia and Inambari endemism centres, respectively east bank and west bank of Rio Madeira. At least eight Birdquest lifer birds were acquired on this tour: the rare Rondonia Bushbird; Brazilian endemics White-breasted Antbird, Manicore Warbling Antbird, Aripuana Antwren and Chico’s Tyrannulet; also Buff-cheeked Tody-Flycatcher, Acre Tody-Tyrant and the amazing Rufous Twistwing. Our itinerary definitely put together one of the finest selections of Amazonian avifauna, though for a next trip there are probably few adjustments to be done. The pre-tour extension campsite brings you to very basic camping conditions, with company of some mosquitoes and relentless heat, but certainly a remarkable site for birding, the Igarapé São João really provided an amazing experience. All other sites 1 BirdQuest Tour Report: Brazil’s Southwestern Amazonia 2017 www.birdquest-tours.com visited on main tour provided considerably easy and very good birding. From the rich east part of Rondonia, the fascinating savannas and endless forests around Humaitá in Amazonas, and finally the impressive bamboo forest at Rio Branco in Acre, this tour focused the endemics from both sides of the medium Rio Madeira. -

Ornithological Surveys in Serranía De Los Churumbelos, Southern Colombia
Ornithological surveys in Serranía de los Churumbelos, southern Colombia Paul G. W . Salaman, Thomas M. Donegan and Andrés M. Cuervo Cotinga 12 (1999): 29– 39 En el marco de dos expediciones biológicos y Anglo-Colombian conservation expeditions — ‘Co conservacionistas anglo-colombianas multi-taxa, s lombia ‘98’ and the ‘Colombian EBA Project’. Seven llevaron a cabo relevamientos de aves en lo Serranía study sites were investigated using non-systematic de los Churumbelos, Cauca, en julio-agosto 1988, y observations and standardised mist-netting tech julio 1999. Se estudiaron siete sitios enter en 350 y niques by the three authors, with Dan Davison and 2500 m, con 421 especes registrados. Presentamos Liliana Dávalos in 1998. Each study site was situ un resumen de los especes raros para cada sitio, ated along an altitudinal transect at c. 300- incluyendo los nuevos registros de distribución más m elevational steps, from 350–2500 m on the Ama significativos. Los resultados estabilicen firme lo zonian slope of the Serranía. Our principal aim was prioridad conservacionista de lo Serranía de los to allow comparisons to be made between sites and Churumbelos, y aluco nos encontramos trabajando with other biological groups (mammals, herptiles, junto a los autoridades ambientales locales con insects and plants), and, incorporating geographi cuiras a lo protección del marcizo. cal and anthropological information, to produce a conservation assessment of the region (full results M e th o d s in Salaman et al.4). A sizeable part of eastern During 14 July–17 August 1998 and 3–22 July 1999, Cauca — the Bota Caucana — including the 80-km- ornithological surveys were undertaken in Serranía long Serranía de los Churumbelos had never been de los Churumbelos, Department of Cauca, by two subject to faunal surveys. -

Ultimate Bolivia Tour Report 2019
Titicaca Flightless Grebe. Swimming in what exactly? Not the reed-fringed azure lake, that’s for sure (Eustace Barnes) BOLIVIA 8 – 29 SEPTEMBER / 4 OCTOBER 2019 LEADER: EUSTACE BARNES Bolivia, indeed, THE land of parrots as no other, but Cotingas as well and an astonishing variety of those much-loved subfusc and generally elusive denizens of complex uneven surfaces. Over 700 on this tour now! 1 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com Blue-throated Macaws hoping we would clear off and leave them alone (Eustace Barnes) Hopefully, now we hear of colourful endemic macaws, raucous prolific birdlife and innumerable elusive endemic denizens of verdant bromeliad festooned cloud-forests, vast expanses of rainforest, endless marshlands and Chaco woodlands, each ringing to the chorus of a diverse endemic avifauna instead of bleak, freezing landscapes occupied by impoverished unhappy peasants. 2 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com That is the flowery prose, but Bolivia IS that great destination. The tour is no longer a series of endless dusty journeys punctuated with miserable truck-stop hotels where you are presented with greasy deep-fried chicken and a sticky pile of glutinous rice every day. The roads are generally good, the hotels are either good or at least characterful (in a good way) and the food rather better than you might find in the UK. The latter perhaps not saying very much. Palkachupe Cotinga in the early morning light brooding young near Apolo (Eustace Barnes). That said, Bolivia has work to do too, as its association with that hapless loser, Che Guevara, corruption, dust and drug smuggling still leaves the country struggling to sell itself. -

Trends in Nectar Concentration and Hummingbird Visitation
SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Fall 2016 Trends in Nectar Concentration and Hummingbird Visitation: Investigating different variables in three flowers of the Ecuadorian Cloud Forest: Guzmania jaramilloi, Gasteranthus quitensis, and Besleria solanoides Sophie Wolbert SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the Animal Studies Commons, Community-Based Research Commons, Environmental Studies Commons, Latin American Studies Commons, and the Plant Biology Commons Recommended Citation Wolbert, Sophie, "Trends in Nectar Concentration and Hummingbird Visitation: Investigating different variables in three flowers of the Ecuadorian Cloud Forest: Guzmania jaramilloi, Gasteranthus quitensis, and Besleria solanoides" (2016). Independent Study Project (ISP) Collection. 2470. https://digitalcollections.sit.edu/isp_collection/2470 This Unpublished Paper is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. Wolbert 1 Trends in Nectar Concentration and Hummingbird Visitation: Investigating different variables in three flowers of the Ecuadorian Cloud Forest: Guzmania jaramilloi, Gasteranthus quitensis, and Besleria solanoides Author: Wolbert, Sophie Academic