Reference ID: 4766120
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insomnia and Anxiety in Older People Sleeping Pills Are Usually Not the Best Solution
® Insomnia and anxiety in older people Sleeping pills are usually not the best solution lmost one-third of older people in the people who take one of United States take sleeping pills. These these medicines sleep medicines are also sometimes called only a little longer and A“sedative-hypnotics” or “tranquilizers.” They better than those who affect the brain and spinal cord. don’t take a medicine. Doctors prescribe some of these medicines Sleeping pills can for sleep problems. Some of these medicines have serious side effects. also can be used to treat other conditions, such All sedative-hypnotic medicines have special as anxiety or alcohol withdrawal. Sometimes, risks for older adults. Seniors are likely to be doctors also prescribe certain anti-depressants more sensitive to the medicines’ effects than for sleep, even though that’s not what they’re younger adults. And these medicines may designed to treat. stay in older people’s bodies longer. These Most older adults should first try to treat their medicines can cause confusion and memory insomnia without medicines. According to the problems that: American Geriatrics Society, there are safer and • Increase the risk of falls and hip fractures. better ways to improve sleep or reduce anxiety. These are common causes of hospital stays Here’s why: and death in older people. Sleeping pills may not help much. • Increase the risk of car accidents. Many ads say that sleeping pills help people get a full, restful night’s sleep. But studies show that this is not exactly true in real life. On average, The new “Z” medicines also have risks. -

Drug & Alcohol Testing Program
Pottawattmie County Drug & Alcohol Testing Program Appendix A Table of Contents POLICY STATEMENT ...................................................................................................................................... 3 SCOPE ............................................................................................................................................................ 4 EDUCATION AND TRAINING .......................................................................................................................... 4 DESIGNATED EMPLOYER REPRESENTATIVE (DER): ....................................................................................... 5 DUTY TO COOPERATE ................................................................................................................................... 5 EMPLOYEE ADMISSION OF ALCOHOL AND CONTROLLED SUBSTANCE USE: (49 CFR Part 382.121) ... 6 PROHIBITED DRUGS AND ILLEGALLY USED CONTROLLED SUBSTANCES: ..................................................... 7 PROHIBITED BEHAVIOR AND CONDUCT: ...................................................................................................... 8 DRUG & ALCOHOL TESTING REQUIREMENTS (49 CFR, Part 40 & 382) ............................................... 10 DRUG & ALCOHOL TESTING CIRCUMSTANCES (49 CFR Part 40 & 382) .............................................. 12 A. Pre-Employment Testing: .................................................................................................... 12 B. Reasonable Suspicion Testing: ......................................................................................... -

Medication Guide Sedative-Hypnotic Capsules
MEDICATION GUIDE SEDATIVE-HYPNOTIC CAPSULES C-II SECONAL SODIUM® CII (secobarbital sodium) CAPSULES, USP Rx only Read this Medication Guide before you start taking a SEDATIVE-HYPNOTIC and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your medical condition or treatment. You and your doctor should talk about the SEDATIVE-HYPNOTIC when you start taking it and at regular checkups. What is the most important information I should know about SEDATIVE-HYPNOTICS? After taking a SEDATIVE-HYPNOTIC, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing. The next morning, you may not remember that you did anything during the night. You have a higher chance for doing these activities if you drink alcohol or take other medicines that make you sleepy with a SEDATIVE-HYPNOTIC. Reported activities include: • driving a car ("sleep-driving") • making and eating food • talking on the phone • having sex • sleep-walking Important: 1. Take SEDATIVE-HYPNOTICS exactly as prescribed • Do not take more SEDATIVE-HYPNOTICS than prescribed. • Take the SEDATIVE-HYPNOTIC right before you get in bed, not sooner. 2. Do not take SEDATIVE-HYPNOTICS if you: • drink alcohol • take other medicines that can make you sleepy. Talk to your doctor about all of your medicines. Your doctor will tell you if you can take SEDATIVE-HYPNOTICS with your other medicines • cannot get a full night's sleep 3. Call your doctor right away if you find out that you have done any of the above activities after taking the SEDATIVE-HYPNOTIC. -

Additional Requested Drugs
PART 1 PART PATIENT PRESCRIBED MEDICATIONS: o ACTIQ o DESIPRAMINE o HYDROCODONE o MORPHINE o ROXICODONE o ADDERALL o DIAZEPAM* o HYDROMORPHONE o MS CONTIN o SOMA o ALPRAZOLAM* o DILAUDID o IMIPRAMINE o NEURONTIN o SUBOXONE o AMBIEN o DURAGESIC o KADIAN o NORCO o TEMAZEPAM o AMITRIPTYLINE o ELAVIL o KETAMINE o NORTRIPTYLINE o TRAMADOL* o ATIVAN o EMBEDA o KLONOPIN o NUCYNTA o TYLENOL #3 o AVINZA o ENDOCET o LORAZEPAM o OPANA o ULTRAM o BUPRENEX o FENTANYL* o LORTAB o OXYCODONE o VALIUM o BUPRENORPHINE o FIORICET o LORCET o OXYCONTIN o VICODIN o BUTRANS o GABAPENTIN o LYRICA o PERCOCET o XANAX o CLONAZEPAM* o GRALISE o METHADONE o RESTORIL TO RE-ORDER CALL RITE-PRINT 718/384-4288 RITE-PRINT CALL TO RE-ORDER ALLIANCE DRUG PANEL (SEE BELOW) o ALCOHOL o BARBITURATES o ILLICITS o MUSCLE RELAXANTS o OPIODS (SYN) ETHANOL PHENOBARBITAL 6-MAM (HEROIN)* CARISPRODOL FENTANYL* BUTABARBITAL a-PVP MEPROBAMATE MEPERIDINE o AMPHETAMINES SECOBARBITAL BENZOYLECGONINE NALOXONE AMPHETAMINE PENTOBARBITAL LSD o OPIODS (NATURAL) METHADONE* METHAMPHETAMINE BUTALBITAL MDA CODEINE METHYLPHENEDATE MDEA MORPHINE o NON-OPIOID o BENZODIAZEPINES MDMA ANALGESICS o OPIODS (SEMI-SYN) o ANTICONVULSIVES ALPRAZOLAM* MDPV TRAMADOL BUPRENORPHINE* GABAPENTIN CLONAZEPAM* MEPHEDRONE TAPENTADOL DIHROCODEINE PREGABALIN DIAZEPAM METHCATHINONE DESOMORPHINE o NON-BENZODIAZEPINE FLUNITRAZEPAM* METHYLONE HYDROCODONE HYPNOTIC o ANTIDEPRESSANTS FLURAZEPAM* PCP HYDROMORPHONE ZOLPIDEM* AMITRIPTYLINE LORAZEPAM THC* OXYCODONE DOXEPIN OXAZEPAM CBD ° OXYMORPHONE o MISCELLANEOUS DRUGS IMIPRIMINE -
![Nembutal [Pentobarbital] Injection and Seconal [Secobarbital] Capsules](https://docslib.b-cdn.net/cover/6349/nembutal-pentobarbital-injection-and-seconal-secobarbital-capsules-686349.webp)
Nembutal [Pentobarbital] Injection and Seconal [Secobarbital] Capsules
Pharmacy Benefit Coverage Criteria Effective Date .......................................... 12/1/2020 Next Review Date… ................................... 12/1/2021 Coverage Policy Number ................................ P0095 Nembutal [pentobarbital] injection and Seconal [secobarbital] capsules Table of Contents Related Coverage Resources Overview .............................................................. 1 Coverage Policy ................................................... 1 FDA Summary ..................................................... 1 General Background ............................................ 2 References .......................................................... 2 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific -

HS 172 R5/13 Briefly Review the Objectives, Content and Activities of This Session
HS 172 R5/13 Briefly review the objectives, content and activities of this session. Upon successfully completing this session the participant will be able to: • Explain a brief history of the CNS Depressant category of drugs. • Identify common drug names and terms associated with this category. • Identify common methods of administration for this category. • Describe the symptoms, observable signs and other effects associated with this category. CONTENT SEGMENTS LEARNING ACTIVITIES A. Overview of the Category Instructor-Led Presentations B. Possible Effects Instructor Led Demonstrations C. OtdDtifEfftOnset and Duration of Effects RdiAiReading Assignmen ts D. Overdose Signs and Symptoms Video Presentations E. Expected Results of the Evaluation Slide Presentations F. Classification Exemplar HS 172 R5/13 9-2 • Explain the typical time parameters, i.e. onset and duration of effects, associated with this category. • List the clues that are likely to emerge when the drug influence evaluation is conducted for a person under the influence of this category of drugs. • Correctly answer the “topics for study” questions at the end of this session. HS 172 R5/13 9-3 A. Overview of the Category CNS Depressants Central Nervous System Depressants slow down the operations of the brain. Point out that other common names for CNS Depressants are “downers” and “sedative-hypnotics.” • Depressants first affect those arareaseas of the brain that control a person’ s conscious, voluntary actions. • Judgment, inhibitions and reaction time are some of the things that CNS Depressants affect first. • As the dose is increased, depressants begin to affect the parts of the brain that control the body’s automatic processes, heartbeat, respiration, etc. -

LITERATURE REVIEW Drug Review
LITERATURE REVIEW Drug Review Document ID: N04-023 Author: Julie Qidwai Date: December 2004 National Advanced Driving Simulator 2401 Oakdale Blvd. Iowa City, IA 52242-5003 Fax (319) 335-4658 TABLE OF CONTENTS 1 Introduction ............................................................................................................................ 1 2 Alprazolam.............................................................................................................................. 1 3 Amitriptyline........................................................................................................................... 2 4 Biperiden................................................................................................................................. 2 5 Brompheniramine .................................................................................................................. 2 6 Butorphanol ............................................................................................................................ 2 7 Cetirizine................................................................................................................................. 3 8 Chloroquine ............................................................................................................................ 3 9 Chlorpheniramine .................................................................................................................. 4 10 Clemastine.............................................................................................................................. -

Midazolam Injection, USP
Midazolam Injection, USP Rx only PHARMACY BULK PACKAGE – NOT FOR DIRECT INFUSION WARNING ADULTS AND PEDIATRICS: Intravenous midazolam has been associated with respiratory depression and respiratory arrest, especially when used for sedation in noncritical care settings. In some cases, where this was not recognized promptly and treated effectively, death or hypoxic encephalopathy has resulted. Intravenous midazolam should be used only in hospital or ambulatory care settings, including physicians’ and dental offices, that provide for continuous monitoring of respiratory and cardiac function, i.e., pulse oximetry. Immediate availability of resuscitative drugs and age- and size-appropriate equipment for bag/valve/mask ventilation and intubation, and personnel trained in their use and skilled in airway management should be assured (see WARNINGS). For deeply sedated pediatric patients, a dedicated individual, other than the practitioner performing the procedure, should monitor the patient throughout the procedures. The initial intravenous dose for sedation in adult patients may be as little as 1 mg, but should not exceed 2.5 mg in a normal healthy adult. Lower doses are necessary for older (over 60 years) or debilitated patients and in patients receiving concomitant narcotics or other central nervous system (CNS) depressants. The initial dose and all subsequent doses should always be titrated slowly; administer over at least 2 minutes and 1 allow an additional 2 or more minutes to fully evaluate the sedative effect. The dilution of the 5 mg/mL formulation is recommended to facilitate slower injection. Doses of sedative medications in pediatric patients must be calculated on a mg/kg basis, and initial doses and all subsequent doses should always be titrated slowly. -

Barbiturates
URINE DRUG TEST INFORMATION SHEET BARBITURATES Classification: Central nervous system depressants insomnia, anxiety or tension due to their very high (CNS Depressants) risk of physical dependence and fatal overdose. Due to the structural nature of barbiturates, the duration Background: Barbiturates are a group of drugs of action does not always correlate well with the that act as central nervous system depressants. biological half-life. Opiates, benzodiazepines and alcohol are also CNS depressants, and like their use, the effect seems to Physiological Effects: Lowered blood pressure, the user as an overall sense of calm. Barbiturates respiratory depression, fatigue, fever, impaired were introduced in 1903, dominating the sedative- coordination, nystagmus, slurred speech and ataxia hypnotic market for the first half of the twentieth century. Unfortunately, because barbiturates have Psychological Effects: Drowsiness, dizziness, unusual a relatively low therapeutic-to-toxic index and excitement, irritability, poor concentration, sedation, substantial potential for abuse, they quickly became confusion, impaired judgment, addiction, euphoria, a major health problem. Barbiturates are commonly decreased anxiety and a loss of inhibition abused for their sedative properties and widespread Toxicity: Barbiturates are especially more availability. The introduction of benzodiazepines in dangerous when abused with alcohol, opiates and the 1960s quickly supplanted the barbiturates due benzodiazepines because they act on the same to their higher safety -
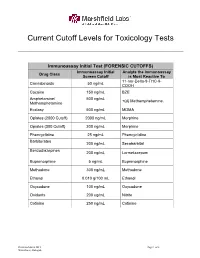
Current Cutoff Levels for Toxicology Tests
Current Cutoff Levels for Toxicology Tests Immunoassay Initial Test (FORENSIC CUTOFFS) Immunoassay Initial Analyte the Immunoassay Drug Class Screen Cutoff is Most Reactive To 11-nor-Delta-9-THC-9- Cannabinoids 50 ng/mL COOH Cocaine 150 ng/mL BZE Amphetamine/ 500 ng/mL +(d) Methamphetamine. Methamphetamine Ecstasy 500 ng/mL MDMA Opiates (2000 Cutoff) 2000 ng/mL Morphine Opiates (300 Cutoff) 300 ng/mL Morphine Phencyclidine 25 ng/mL Phencyclidine Barbiturates 200 ng/mL Secobarbital Benzodiazepines 200 ng/mL Lormetazepam Buprenorphine 5 ng/mL Buprenorphine Methadone 300 ng/mL Methadone Ethanol 0.010 g/100 mL Ethanol Oxycodone 100 ng/mL Oxycodone Oxidants 200 ug/mL Nitrite Cotinine 250 ng/mL Cotinine Revision March 2015 Page 1 of 3 Winterhack, Flanagan Critical Value Testing* Test Cutoff Analyte(s) Tested For Critical/Action Level Ethylene Glycol 10 mg/dL Ethylene Glycol 20 mg/dL Ethanol, Methanol, Methanol: 0.020 g/100 mL Volatiles 0.010 g/100 mL Acetone, Isopropanol Isopropanol: 0.400 g/100 mL Forensic Workplace Confirmation Testing Cutoffs (GC-MS, GC, LCMSMS, or LCMS QToF Methods) Drug Class Cutoff Analyte(s) Confirmed Cannabinoids 15 ng/mL Delta-9-THC-COOH Cocaine 100 ng/mL BZE (Benzoylecgonine) Amphetamine, Methamphetamine, MDA, MDMA Amphetamines 250 ng/mL (Ecstasy) Opiates (2000 Cutoff) 2000 ng/mL Codeine and Morphine Codeine, Morphine, Hydrocodone, and Opiates (300 Cutoff) 300 ng/mL Hydromorphone Phencyclidine 25 ng/mL Phencyclidine Butalbital, Amobarbital, Pentobarbital, Barbiturates 300 ng/mL Secobarbital, and Phenobarbital 300 -

Investigations in Fish Control
INVESTIGATIONS IN FISH CONTROL 29. Efficacy of Methylpentynol as an Anesthetic on Four Salmonids 30. Toxicity of Methylpentynol to Selected Fishes 31. Annotated Bibliography on Methylpentynol United States Department of the Interior Fish and Wildlife Service Bureau of Sport Fisheries and Wildlife INVESTIGATIONS IN FISH CONTROL Investigations in Fish Control, published by the Bureau of Sport Fisheries and Wildlife, in clude reports on the results of work at the Bureau's Fish Control Laboratories at La Crosse, Wis., and Warm Springs, Ga., and reports of other studies related to that work. Though each report is regarded as a separate publication, several may be issued under a single cover, for economy. Current reports in this series are (Reports 1 and 2 are in one cover.) 1. Laboratories and Methods for Screening Fish-Control Chemicals, by Robert E. Lennon and Charles R. Walker. 1964. 15 p. 2. Preliminary Observations on the Toxicity of Antimycin A to Fish and Other Aquatic Animals, by Charles R. Walker, Robert E. Lennon, and Bernard L. Berger. 1964. 18 p. (Reports 3 through 8 are in one cover.) 3. Minimum Lethal Levels of Toxaphene as a Piscicide in North Dakota Lakes, by Dale L. Henegar. 1966. 16 p. 4. Effects of Toxaphene on Plankton and Aquatic Invertebrates in North Dakota Lakes, by Robert G. Needham. 1966. 16 p. 5. Growth Rates of Yellow Perch in Two North Dakota Lakes After Population Reduction with Toxaphene, by Donald C. Warnick. 1966. 9 p. 6. Mortality of Some Species of Fish to Toxaphene at Three Temperatures, by Mahmoud Ahmed Mahdi. -

Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: an American Academy of Sleep Medicine Clinical Practice Guideline Michael J
pii: jc-00382-16 http://dx.doi.org/10.5664/jcsm.6470 SPECIAL ARTICLES Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline Michael J. Sateia, MD1; Daniel J. Buysse, MD2; Andrew D. Krystal, MD, MS3; David N. Neubauer, MD4; Jonathan L. Heald, MA5 1Geisel School of Medicine at Dartmouth, Hanover, NH; 2University of Pittsburgh School of Medicine, Pittsburgh, PA; 3University of California, San Francisco, San Francisco, CA; 4Johns Hopkins University School of Medicine, Baltimore, MD; 5American Academy of Sleep Medicine, Darien, IL Introduction: The purpose of this guideline is to establish clinical practice recommendations for the pharmacologic treatment of chronic insomnia in adults, when such treatment is clinically indicated. Unlike previous meta-analyses, which focused on broad classes of drugs, this guideline focuses on individual drugs commonly used to treat insomnia. It includes drugs that are FDA-approved for the treatment of insomnia, as well as several drugs commonly used to treat insomnia without an FDA indication for this condition. This guideline should be used in conjunction with other AASM guidelines on the evaluation and treatment of chronic insomnia in adults. Methods: The American Academy of Sleep Medicine commissioned a task force of four experts in sleep medicine. A systematic review was conducted to identify randomized controlled trials, and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process was used to assess the evidence. The task force developed recommendations and assigned strengths based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.