(12) Patent Application Publication (10) Pub. No.: US 2007/0270403 A1 Broderick (43) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2010/0179214 A1 Dubé Et Al
US 20100179214A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0179214 A1 Dubé et al. (43) Pub. Date: Jul. 15, 2010 (54) DOXEPIN TRANS ISOMERS AND SOMERC continuation-in-part of application No. 1 1/804,720, MIXTURES AND METHODS OF USING THE filed on May 18, 2007. SAME TO TREAT SLEEP DSORDERS (60) Provisional application No. 60/898,378, filed on Jan. (75) Inventors: Susan E. Dubé, Carlsbad, CA (US); 30, 2007, provisional application No. 60/801,824, Neil B. Kavey, Chappaqua, NY filed on May 19, 2006, provisional application No. (US) 60/833,319, filed on Jul 25, 2006. Correspondence Address: KNOBBE MARTENS OLSON & BEAR LLP Publication Classification 2040 MAINSTREET, FOURTEENTH FLOOR (51) Int. Cl. IRVINE, CA 92.614 (US) A63L/335 (2006.01) A6IP 25/20 (2006.01) (73) Assignee: SOMAXON PHARMACEUTICALS, INC., (52) U.S. Cl. ........................................................ S14/450 San Diego, CA (US) (21) Appl. No.: 12/535,623 (57) ABSTRACT The invention relates to use of the trans-(E) isomer or iso (22) Filed: Aug. 4, 2009 meric mixtures containing specified ratios of the trans-(E) and cis-(Z) isomers of doxepin, metabolites of doxepin, phar Related U.S. Application Data maceutically-acceptable salts of doxepin and prodrugs of the (63) Continuation-in-part of application No. 12/022,788, same; compositions containing the same, for the treatment of filed on Jan. 30, 2008, now abandoned, which is a sleep disorders US 2010/0179214 A1 Jul. 15, 2010 DOXEPIN TRANS ISOMERS AND SOMERC brings scrutiny from the Drug Enforcement Administration MIXTURES AND METHODS OF USING THE and other regulatory bodies, and requires registration and SAME TO TREAT SLEEP DSORDERS administrative controls in physicians offices. -

List of Vital Essential and Necessary Drugs and Medical Sundries For
LIST OF VITAL ESSENTIAL AND NECESSARY DRUGS AND 2015 MEDICAL SUNDRIES FOR PUBLIC HEALTH INSTITUTIONS Sixth Edition STANDARDS & REGULATION DIVISION JAMAICA List of Vital Essential and Necessary List of Drugs and Medical Sundries for Public Institutions List of Vital Essential and Necessary List of Drugs and Medical Sundries for Public Institutions CONTENTS CONTENTS Contd. Page Preface 5-6 Page Information on Hospitals and Health Centres 7 Explanatory Notes 8 Medical Sundries 69-73 Prescription Writing 9-10 Dental Supplies 74 Algorithm for Treatment of Hypertension 11-12 Radiotherapy – Diagnostic Agents 75 Algorithm for Management of Type 2 Diabetes 13-14 Raw Materials 76 List of Drugs Designated for NHF 15-17 List of Drugs Designated for JADEP 18 VOLUME11 – SPECIALIST LIST 77 VOLUME 1 – GENERAL LIST 19 CLASSIFICATION OF DRUGS SECTION 1. Cardiovascular System 78 CLASSIFICATION OF DRUGS SECTION 2. Central Nervous System 79 SECTION 1. Cardiovascular System 20-24 SECTION 3. Dermatology 80 SECTION 2. Central Nervous System 25-30 SECTION 4. Endocrine System 80 SECTION 3. Dermatology 31-33 SECTION 5. Gastro-intestinal System 81 SECTION 4. Ear, Nose and Oropharynx 34-35 SECTION 6. Infections 81 SECTION 5. Endocrine System 36-38 SECTION 7. Malignant Disease and SECTION 6. Gastro-intestinal System 39-40 Immunosuppression 82 SECTION 7. Infections 41-46 SECTION 8. Musculoskeletal and Joint Diseases 83 SECTION 8. Malignant Disease and SECTION 9. Ophthalmology 83 Immunosuppression 47-49 SECTION 10. Genito-Urinary Tract Disorders 84 SECTION 9. Musculoskeletal and Joint Diseases 50-51 SECTION 11. Respiratory System 84 SECTION 10. Nutrition and Blood 52-54 SECTION 12. -

Copyrighted Material
Index Note: page numbers in italics refer to figures; those in bold to tables or boxes. abacavir 686 tolerability 536–537 children and adolescents 461 acamprosate vascular dementia 549 haematological 798, 805–807 alcohol dependence 397, 397, 402–403 see also donepezil; galantamine; hepatic impairment 636 eating disorders 669 rivastigmine HIV infection 680 re‐starting after non‐adherence 795 acetylcysteine (N‐acetylcysteine) learning disability 700 ACE inhibitors see angiotensin‐converting autism spectrum disorders 505 medication adherence and 788, 790 enzyme inhibitors obsessive compulsive disorder 364 Naranjo probability scale 811, 812 acetaldehyde 753 refractory schizophrenia 163 older people 525 acetaminophen, in dementia 564, 571 acetyl‐L‐carnitine 159 psychiatric see psychiatric adverse effects acetylcholinesterase (AChE) 529 activated partial thromboplastin time 805 renal impairment 647 acetylcholinesterase (AChE) acute intoxication see intoxication, acute see also teratogenicity inhibitors 529–543, 530–531 acute kidney injury 647 affective disorders adverse effects 537–538, 539 acutely disturbed behaviour 54–64 caffeine consumption 762 Alzheimer’s disease 529–543, 544, 576 intoxication with street drugs 56, 450 non‐psychotropics causing 808, atrial fibrillation 720 rapid tranquillisation 54–59 809, 810 clinical guidelines 544, 551, 551 acute mania see mania, acute stupor 107, 108, 109 combination therapy 536 addictions 385–457 see also bipolar disorder; depression; delirium 675 S‐adenosyl‐l‐methionine 275 mania dosing 535 ADHD -

A Phase 3, Multicenter, Randomized, Double-Blind
Official Title: A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of Adjunctive Pimavanserin in Subjects With Major Depressive Disorder and Inadequate Response to Antidepressant Treatment NCT Number: NCT03999918 Document Date: 21 Jun 2020 CLINICAL STUDY PROTOCOL UNMASKED PROTOCOL A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of Adjunctive Pimavanserin in Subjects With Major Depressive Disorder and Inadequate Response to Antidepressant Treatment Protocol Number: ACP-103-054 Amendment 4 EudraCT Number: 2018-003251-37 Original Protocol Date: 30 August 2018 Protocol Amendment 1 Date: 05 December 2018 Protocol Amendment 2 Date: 18 March 2019 Protocol Amendment 3 Date: 29 October 2019 Protocol Amendment 4 Date: 21 June 2020 Confidentiality Statement This protocol is the confidential information of ACADIA Pharmaceuticals Inc. and is intended solely for the guidance of the clinical investigation. This protocol may not be disclosed to parties not associated with the clinical investigation or used for any purpose without the prior written consent of ACADIA Pharmaceuticals Inc. Confidential and Proprietary Information of ACADIA Pharmaceuticals Inc. Page 1 of 81 Study: ACP-103-054 Final Version: 1.0 Clinical Study Protocol Amendment 4 UNMASKED VERSION Date: 21 June 2020 SPONSOR SIGNATURE PAGE Title: A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of Adjunctive Pimavanserin in Subjects With Major Depressive Disorder and Inadequate Response to Antidepressant Treatment ACADIA President: President ACADIA Pharmaceuticals Inc. See appended electronic signature page Signature Date ACADIA Study Lead: Executive Director, Clinical Research ACADIA Pharmaceuticals Inc. -

Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans
molecules Review Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans Paul Cumming 1,2,* , Milan Scheidegger 3 , Dario Dornbierer 3, Mikael Palner 4,5,6 , Boris B. Quednow 3,7 and Chantal Martin-Soelch 8 1 Department of Nuclear Medicine, Bern University Hospital, CH-3010 Bern, Switzerland 2 School of Psychology and Counselling, Queensland University of Technology, Brisbane 4059, Australia 3 Department of Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital of the University of Zurich, CH-8032 Zurich, Switzerland; [email protected] (M.S.); [email protected] (D.D.); [email protected] (B.B.Q.) 4 Odense Department of Clinical Research, University of Southern Denmark, DK-5000 Odense, Denmark; [email protected] 5 Department of Nuclear Medicine, Odense University Hospital, DK-5000 Odense, Denmark 6 Neurobiology Research Unit, Copenhagen University Hospital, DK-2100 Copenhagen, Denmark 7 Neuroscience Center Zurich, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8058 Zurich, Switzerland 8 Department of Psychology, University of Fribourg, CH-1700 Fribourg, Switzerland; [email protected] * Correspondence: [email protected] or [email protected] Abstract: Hallucinogens are a loosely defined group of compounds including LSD, N,N- dimethyltryptamines, mescaline, psilocybin/psilocin, and 2,5-dimethoxy-4-methamphetamine (DOM), Citation: Cumming, P.; Scheidegger, which can evoke intense visual and emotional experiences. We are witnessing a renaissance of re- M.; Dornbierer, D.; Palner, M.; search interest in hallucinogens, driven by increasing awareness of their psychotherapeutic potential. Quednow, B.B.; Martin-Soelch, C. As such, we now present a narrative review of the literature on hallucinogen binding in vitro and Molecular and Functional Imaging ex vivo, and the various molecular imaging studies with positron emission tomography (PET) or Studies of Psychedelic Drug Action in single photon emission computer tomography (SPECT). -

Structure, Function, and Pharmaceutical Ligands of 5-Hydroxytryptamine 2B Receptor
pharmaceuticals Review Structure, Function, and Pharmaceutical Ligands of 5-Hydroxytryptamine 2B Receptor Qing Wang 1,2 , Yu Zhou 2 , Jianhui Huang 1 and Niu Huang 2,3,* 1 School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China; [email protected] (Q.W.); [email protected] (J.H.) 2 National Institute of Biological Sciences, No. 7 Science Park Road, Zhongguancun Life Science Park, Beijing 102206, China; [email protected] 3 Tsinghua Institute of Multidisciplinary Biomedical Research, Tsinghua University, Beijing 102206, China * Correspondence: [email protected]; Tel.: +86-10-80720645 Abstract: Since the first characterization of the 5-hydroxytryptamine 2B receptor (5-HT2BR) in 1992, significant progress has been made in 5-HT2BR research. Herein, we summarize the biological function, structure, and small-molecule pharmaceutical ligands of the 5-HT2BR. Emerging evidence has suggested that the 5-HT2BR is implicated in the regulation of the cardiovascular system, fibrosis disorders, cancer, the gastrointestinal (GI) tract, and the nervous system. Eight crystal complex structures of the 5-HT2BR bound with different ligands provided great insights into ligand recognition, activation mechanism, and biased signaling. Numerous 5-HT2BR antagonists have been discovered and developed, and several of them have advanced to clinical trials. It is expected that the novel 5-HT2BR antagonists with high potency and selectivity will lead to the development of first-in-class drugs in various therapeutic areas. Keywords: GPCR; 5-HT2BR; biased signaling; agonist; antagonist Citation: Wang, Q.; Zhou, Y.; Huang, J.; Huang, N. Structure, Function, and Pharmaceutical Ligands of 5-Hydroxytryptamine 2B Receptor. 1. Introduction Pharmaceuticals 2021, 14, 76. -

Review Article Monoamine Reuptake Inhibitors in Parkinson's Disease
Review Article Monoamine Reuptake Inhibitors in Parkinson’s Disease Philippe Huot,1,2,3 Susan H. Fox,1,2 and Jonathan M. Brotchie1 1 Toronto Western Research Institute, Toronto Western Hospital, University Health Network, 399 Bathurst Street, Toronto, ON, Canada M5T 2S8 2Division of Neurology, Movement Disorder Clinic, Toronto Western Hospital, University Health Network, University of Toronto, 399BathurstStreet,Toronto,ON,CanadaM5T2S8 3Department of Pharmacology and Division of Neurology, Faculty of Medicine, UniversitedeMontr´ eal´ and Centre Hospitalier de l’UniversitedeMontr´ eal,´ Montreal,´ QC, Canada Correspondence should be addressed to Jonathan M. Brotchie; [email protected] Received 19 September 2014; Accepted 26 December 2014 Academic Editor: Maral M. Mouradian Copyright © 2015 Philippe Huot et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The motor manifestations of Parkinson’s disease (PD) are secondary to a dopamine deficiency in the striatum. However, the degenerative process in PD is not limited to the dopaminergic system and also affects serotonergic and noradrenergic neurons. Because they can increase monoamine levels throughout the brain, monoamine reuptake inhibitors (MAUIs) represent potential therapeutic agents in PD. However, they are seldom used in clinical practice other than as antidepressants and wake-promoting agents. This review -
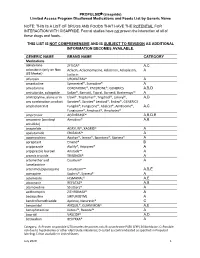
PROPULSID® (Cisapride) Limited Access Program
PROPULSID (cisapride) Limited Access Program Disallowed Medications and Foods List by Generic Name NOTE: THIS IS A LIST OF DRUGS AND FOODS THAT HAVE THE POTENTIAL FOR INTERACTION WITH CISAPRIDE. Formal studies have not proven the interaction of all of these drugs and foods. THIS LIST IS NOT COMPREHENSIVE AND IS SUBJECT TO REVISION AS ADDITIONAL INFORMATION BECOMES AVAILABLE. GENERIC NAME BRAND NAME CATEGORY Medications abiraterone ZYTIGA® A,C aclarubicin (only on Non Aclacin, Aclacinomycine, Aclacinon, Aclaplastin, A US Market) Jaclacin alfuzosin UROXATRAL® A amantadine Symmetrel®, Symadine® A amiodarone CORDARONE®, PACERONE®, GENERICS A,B,D amisulpride, sultopride Solian®, Barnotil, Topral, Barnetil, Barhemsys™ A amitriptyline, alone or in Elavil®, Tryptomer®, Tryptizol®, Laroxyl®, A,D any combination product Saroten®, Sarotex® Lentizol®, Endep®, GENERICS amphotericin B Fungilin®, Fungizone®, Abelcet®, AmBisome®, A,C Fungisome®, Amphocil®, Amphotec® amprenavir AGENERASE® A,B,D,E amsacrine (acridinyl Amsidine® A,B anisidide) anagrelide AGRYLIN®, XAGRID® A apalutamide ERLEADA™ A apomorphine Apokyn®, Ixense®, Spontane®, Uprima® A aprepitant Emend® B aripiprazole Abilify®, Aripiprex® A aripiprazole lauroxil Aristada™ A arsenic trioxide TRISENOX® A artemether and Coartem® A lumefantrine artenimol/piperaquine Eurartesim™ A,B,E asenapine Saphris®, Sycrest® A astemizole HISMANAL® A,E atazanavir REYATAZ® A,B atomoxetine Strattera® A azithromycin ZITHROMAX® A bedaquiline SIRTURO(TM) A bendroflumethiazide Aprinox, Naturetin® C benperidol ANQUIL®, -

5-HT2A Receptors in the Central Nervous System the Receptors
The Receptors Bruno P. Guiard Giuseppe Di Giovanni Editors 5-HT2A Receptors in the Central Nervous System The Receptors Volume 32 Series Editor Giuseppe Di Giovanni Department of Physiology & Biochemistry Faculty of Medicine and Surgery University of Malta Msida, Malta The Receptors book Series, founded in the 1980’s, is a broad-based and well- respected series on all aspects of receptor neurophysiology. The series presents published volumes that comprehensively review neural receptors for a specific hormone or neurotransmitter by invited leading specialists. Particular attention is paid to in-depth studies of receptors’ role in health and neuropathological processes. Recent volumes in the series cover chemical, physical, modeling, biological, pharmacological, anatomical aspects and drug discovery regarding different receptors. All books in this series have, with a rigorous editing, a strong reference value and provide essential up-to-date resources for neuroscience researchers, lecturers, students and pharmaceutical research. More information about this series at http://www.springer.com/series/7668 Bruno P. Guiard • Giuseppe Di Giovanni Editors 5-HT2A Receptors in the Central Nervous System Editors Bruno P. Guiard Giuseppe Di Giovanni Faculté de Pharmacie Department of Physiology Université Paris Sud and Biochemistry Université Paris-Saclay University of Malta Chatenay-Malabry, France Msida MSD, Malta Centre de Recherches sur la Cognition Animale (CRCA) Centre de Biologie Intégrative (CBI) Université de Toulouse; CNRS, UPS Toulouse, France The Receptors ISBN 978-3-319-70472-2 ISBN 978-3-319-70474-6 (eBook) https://doi.org/10.1007/978-3-319-70474-6 Library of Congress Control Number: 2017964095 © Springer International Publishing AG 2018 This work is subject to copyright. -

Anti Leishmanial Activities of Some Antidepressant Drugs, and Antifungal Drugs
Journal of Advance Research in Pharmacy & Biological Science ISSN: 2208-2360 Anti Leishmanial Activities of Some Antidepressant Drugs, And Antifungal Drugs. Sagar S Trivedi*, Ms Geeta.N.Lodhi, Dr.C.A .Doifode, Harsha Raut. Shri Sachhidanand shikshan Sansthan’s Taywade College of Pharmacy ,Koradi. ABSRACT Leishmanisis is wide range, worldwide, without drug, vaccine, secticide and has not sterile immunity and efforts in this field have not been successful.Leishmaniasis is a complex vector- borne disease caused by different species of Leishmania. It affects at least 12 million people worldwide. Leishmaniasis is commonly associated with poor economic conditions and immune compromised situations like HIV co infection. There is increasing in drug resistance to commonly used therapeutics as well as lack of vaccine program. This leads to a perpetual search for a new drug for leishmaniasis. This review fundamentally deals with some antidepressant drugs, and antifugal drugs showing the anti leishmanial activities. The antidepressant drugs are Imipramine, Sertraline, Ketanserin and Mianserin. Imipramine being a Tri Cyclic Antidepressant (TCA) drug kills Leishmania donovani elevating IL-12/IL-10 ratio. Sertraline belonging to the selective serotonin reuptake inhibitor (SSRI) drugs, removes parasite loads from spleen and liver probably by declining cytoplasmic ATP consumption. Ketanserin is a serotonin receptor (5- HT2A/2C) antagonist that kills both amastigotes and promastigotes probably due to inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR). Mianserin belonging to the TCA group of drug, kills both promastigote and amastigote parasites also probably due to the inhibition of HMGR. The present review will give the summarized information about putting some old antidepressant drugs for the treatment of another disease like Leishmania. -

( 19 ) United States
USOORE48059E (19 ) United States ( 12 ) Reissued Patent (10 ) Patent Number : US RE48,059 E Yamashita et al. ( 45 ) Date of Reissued Patent: * Jun . 23 , 2020 (54 ) PIPERAZINE -SUBSTITUTED 4,734,416 A 3/1988 Banno et al . BENZOTHIOPHENES FOR TREATMENT OF 4,824,840 A 4/1989 Banno et al. 4,831,031 A 5/1989 Lowe, III et al . MENTAL DISORDERS 4,847,254 A 7/1989 Boegesoe et al . 4,883,795 A 11/1989 Lowe, III et al . ( 71) Applicant: Otsuka Pharmaceutical Co., Ltd., 5,006,528 A 4/1991 Oshiro et al . Tokyo (JP ) 5,424,313 A 6/1995 Hartog et al . 5,436,246 A * 7/1995 Bernotas et al . 514 / 252.13 5,506,248 A 4/1996 Nikfar et al . ( 72 ) Inventors : Hiroshi Yamashita , Tokushima ( JP ) ; 5,753,661 A 5/1998 Moltzen et al. Nobuaki Ito , Tokushima ( JP ) ; Shin 5,846,982 A 12/1998 Audia et al. Miyamura , Tokushima (JP ) ; Kunio 5,919,485 A 7/1999 Cochran et al. Oshima, Tokushima (JP ) ; Jun 5,958,924 A 9/1999 McCort et al . 5,977,110 A 11/1999 Belliotti et al . Matsubara , Tokushima ( JP ) ; Hideaki 6,140,331 A 10/2000 Moltzen et al . Kuroda , Tokushima (JP ) ; Haruka 6,187,340 B1 2/2001 Fukuta et al . Takahashi, Tokushima ( JP ) ; Satoshi 6,214,829 B1 4/2001 Feenstra et al. Shimizu , Tokushima ( JP ) ; Tatsuyoshi 6,225,312 B1 5/2001 Feenstra et al. Tanaka , Tokushima ( JP ) ; Yasuo 6,262,056 B1 7/2001 Monge Vega et al . 6,562,375 B1 5/2003 Sako et al. -

Typical and Atypical Antipsychotic Drugs Increase Extracellular Histamine Levels in the Rat Medial Prefrontal Cortex: Contribution of Histamine H1 Receptor Blockade
ORIGINAL RESEARCH ARTICLE published: 17 May 2012 PSYCHIATRY doi: 10.3389/fpsyt.2012.00049 Typical and atypical antipsychotic drugs increase extracellular histamine levels in the rat medial prefrontal cortex: contribution of histamine H1 receptor blockade Matthew J. Fell 1†, Jason S. Katner 1, Kurt Rasmussen1, Alexander Nikolayev 2, Ming-Shang Kuo2, David L. G. Nelson1, Kenneth W. Perry 1 and Kjell A. Svensson1* 1 Psychiatric Disorders, Neuroscience Discovery Research, Eli Lilly and Company, Indianapolis, IN, USA 2 Translational Science, Eli Lilly and Company, Indianapolis, IN, USA Edited by: Atypical antipsychotics such as clozapine and olanzapine have been shown to enhance Bernat Kocsis, Harvard Medical histamine turnover and this effect has been hypothesized to contribute to their improved School, USA therapeutic profile compared to typical antipsychotics. In the present study, we examined Reviewed by: Francesc Artigas, Instituto de the effects of antipsychotic drugs on histamine (HA) efflux in the mPFC of the rat by means Investigaciones Biomédicas de of in vivo microdialysis and sought to differentiate the receptor mechanisms which under- Barcelona, Spain lie such effects. Olanzapine and clozapine increased mPFC HA efflux in a dose related Ritchie Brown, Harvard Medical manner. Increased HA efflux was also observed after quetiapine, chlorpromazine, and per- School, USA phenazine treatment. We found no effect of the selective 5-HT2A antagonist MDL100907, *Correspondence: Kjell A. Svensson, Neuroscience 5-HT2c antagonist SB242084, or the 5-HT6 antagonist Ro 04-6790 on mPFC HA efflux. Discovery Research, Lilly Research HA efflux was increased following treatment with selective H1 receptor antagonists pyril- Laboratories, Lilly Corporate Center, amine, diphenhydramine, and triprolidine, the H3 receptor antagonist ciproxifan and the Indianapolis, IN 46285, USA.