The Elucidation of Stress Memory Inheritance in Brassica Rapa Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
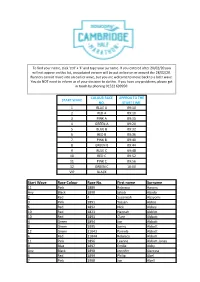
Start Wave Race Colour Race No. First Name Surname
To find your name, click 'ctrl' + 'F' and type your surname. If you entered after 20/02/20 you will not appear on this list, an updated version will be put online on or around the 28/02/20. Runners cannot move into an earlier wave, but you are welcome to move back to a later wave. You do NOT need to inform us of your decision to do this. If you have any problems, please get in touch by phoning 01522 699950. COLOUR RACE APPROX TO THE START WAVE NO. START TIME 1 BLUE A 09:10 2 RED A 09:10 3 PINK A 09:15 4 GREEN A 09:20 5 BLUE B 09:32 6 RED B 09:36 7 PINK B 09:40 8 GREEN B 09:44 9 BLUE C 09:48 10 RED C 09:52 11 PINK C 09:56 12 GREEN C 10:00 VIP BLACK Start Wave Race Colour Race No. First name Surname 11 Pink 1889 Rebecca Aarons Any Black 1890 Jakob Abada 2 Red 4 Susannah Abayomi 3 Pink 1891 Yassen Abbas 6 Red 1892 Nick Abbey 10 Red 1823 Hannah Abblitt 10 Red 1893 Clare Abbott 4 Green 1894 Jon Abbott 8 Green 1895 Jonny Abbott 12 Green 11043 Pamela Abbott 6 Red 11044 Rebecca Abbott 11 Pink 1896 Leanne Abbott-Jones 9 Blue 1897 Emilie Abby Any Black 1898 Jennifer Abecina 6 Red 1899 Philip Abel 7 Pink 1900 Jon Abell 10 Red 600 Kirsty Aberdein 6 Red 11045 Andrew Abery Any Black 1901 Erwann ABIVEN 11 Pink 1902 marie joan ablat 8 Green 1903 Teresa Ablewhite 9 Blue 1904 Ahid Abood 6 Red 1905 Alvin Abraham 9 Blue 1906 Deborah Abraham 6 Red 1907 Sophie Abraham 1 Blue 11046 Mitchell Abrams 4 Green 1908 David Abreu 11 Pink 11047 Kathleen Abuda 10 Red 11048 Annalisa Accascina 4 Green 1909 Luis Acedo 10 Red 11049 Vikas Acharya 11 Pink 11050 Catriona Ackermann -

82755929.Pdf
FEBS Letters 589 (2015) 2931–2943 journal homepage: www.FEBSLetters.org Review The 4D nucleome: Evidence for a dynamic nuclear landscape based on co-aligned active and inactive nuclear compartments ⇑ Thomas Cremer a, , Marion Cremer a, Barbara Hübner a,1, Hilmar Strickfaden b, Daniel Smeets a, ⇑ ⇑ Jens Popken a, Michael Sterr a,2, Yolanda Markaki a, Karsten Rippe c, , Christoph Cremer d, a Biocenter, Department Biology II, Ludwig Maximilians University (LMU), Martinsried, Germany b University of Alberta, Cross Cancer Institute Dept. of Oncology, Edmonton, AB, Canada c German Cancer Research Center (DKFZ) & BioQuant Center, Research Group Genome Organization & Function, Heidelberg, Germany d Institute of Molecular Biology (IMB), Mainz and Institute of Pharmacy and Molecular Biotechnology (IPMB), University of Heidelberg, Germany article info abstract Article history: Recent methodological advancements in microscopy and DNA sequencing-based methods provide Received 1 April 2015 unprecedented new insights into the spatio-temporal relationships between chromatin and nuclear Revised 19 May 2015 machineries. We discuss a model of the underlying functional nuclear organization derived mostly Accepted 20 May 2015 from electron and super-resolved fluorescence microscopy studies. It is based on two spatially Available online 28 May 2015 co-aligned, active and inactive nuclear compartments (ANC and INC). The INC comprises the com- Edited by Wilhelm Just pact, transcriptionally inactive core of chromatin domain clusters (CDCs). The ANC is formed by the transcriptionally active periphery of CDCs, called the perichromatin region (PR), and the inter- chromatin compartment (IC). The IC is connected to nuclear pores and serves nuclear import and Keywords: 4D nucleome export functions. The ANC is the major site of RNA synthesis. -

Warning Sines: Alu Elements, Evolution of the Human Brain, and the Spectrum of Neurological Disease
Chromosome Res (2018) 26:93–111 https://doi.org/10.1007/s10577-018-9573-4 ORIGINAL ARTICLE Warning SINEs: Alu elements, evolution of the human brain, and the spectrum of neurological disease Peter A. Larsen & Kelsie E. Hunnicutt & Roxanne J. Larsen & Anne D. Yoder & Ann M. Saunders Received: 6 December 2017 /Revised: 14 January 2018 /Accepted: 15 January 2018 /Published online: 19 February 2018 # The Author(s) 2018. This article is an open access publication Abstract Alu elements are a highly successful family of neurological networks are potentially vulnerable to the primate-specific retrotransposons that have fundamen- epigenetic dysregulation of Alu elements operating tally shaped primate evolution, including the evolution across the suite of nuclear-encoded mitochondrial genes of our own species. Alus play critical roles in the forma- that are critical for both mitochondrial and CNS func- tion of neurological networks and the epigenetic regu- tion. Here, we highlight the beneficial neurological as- lation of biochemical processes throughout the central pects of Alu elements as well as their potential to cause nervous system (CNS), and thus are hypothesized to disease by disrupting key cellular processes across the have contributed to the origin of human cognition. De- CNS. We identify at least 37 neurological and neurode- spite the benefits that Alus provide, deleterious Alu generative disorders wherein deleterious Alu activity has activity is associated with a number of neurological been implicated as a contributing factor for the manifes- and neurodegenerative disorders. In particular, tation of disease, and for many of these disorders, this activity is operating on genes that are essential for proper mitochondrial function. -

Family Name Files That Can Be Accessed in the Boerne Public Library Local and Family History Archives
FAMILY NAME FILES THAT CAN BE ACCESSED IN THE BOERNE PUBLIC LIBRARY LOCAL AND FAMILY HISTORY ARCHIVES A Abadie, Ables, Abshire Ackerman Adair, Adam, Adamek, Adamietz, Adams, Adamson, Adcock, Adkins, Adler, Adrian, Adriance, Agold, Aguirre, Ahr, Ahrlett, Alba, Albrecht, Albright, Alcorn, Alderman, Aldridge, Alexander, Alf, Alford, Algueseva, Allamon, Allen, Allison, Altgelt, Alvarez, Amason, Ammann, Ames, Anchondo, Anderson, Angell, Angle, Ansaldo, Anstiss, Appelt, Armendarez, Armstrong, Arnold, Arnott, Artz, Ashcroft, Asher, Atencio, Atkinson, Aue, Austin, Aycock, B Backer, Bacon, Bacorn, Bain, Baker, Baldwin, Ball, Balser, Bangert, Bankier, Banks, Bannister, Barbaree, Barker, Barkley, Barnard, Barnes, Barnett, Barnette, Barnhart, Baron, Barrett, Barrington, Barron, Barrows, Barta, Barteau, Bartel, Bartels, Barthlow, Barton, Basham, Bates, Batha, Bauer, Baum, Baumann, Bausch, Baxter, Bayard, Bayless, Baynton, Beagle, Bealor, Beam, Bean, Beardslee, Beasley, Beath, Beatty, Beauford, Beaver, Bechtold, Beck, Becker, Beckett, Beckley, Bedford, Bedgood, Bednar, Bedwell, Beem, Beene, Beer, Begia, Behr, Beissner, Bell, Below, Bemus, Benavides, Bender, Bennack, Benefield, Benner, Bennett, Benson, Bentley, Berger, Bergmann, Berlin, Berline, Bernal, Berne, Bernert, Bernhard, Bernstein, Berry, Besch, Beseler, Beshea, Besser, Best, Bettison, Beutnagel, Bevers, Bey, Beyer, Bickel, Bidus, Bien, Bierman, Bierschwale, Bigger, Biggs, Billingsley, Birdsong, Birkner, Bish, Bitzkie, Black, Blackburn, Blackford, Blackwell, Blair, Blaize, Blake, Blakey, Blalock, -

Germanic Surname Lexikon
Germanic Surname Lexikon Most Popular German Last Names with English Meanings German for Beginners: Contents - Free online German course Introduction German Vocabulary: English-German Glossaries For each Germanic surname in this databank we have provided the English Free Online Translation To or From German meaning, which may or may not be a surname in English. This is not a list of German for Beginners: equivalent names, but rather a sampling of English translations of German names. Das Abc German for Beginners: In many cases, there may be several possible origins or translations for a Lektion 1 surname. The translation shown for a surname may not be the only possibility. Some names are derived from Old German and may have a different meaning from What's Hot that in modern German. Name research is not always an exact science. German Word of the Day - 25. August German Glossary of Abbreviations: OHG (Old High German) Words to Avoid - T German Words to Avoid - Glossary Warning German Glossary of Germanic Last Names (A-K) Words to Avoid - With English Meanings Feindbilder German Words to Avoid - Nachname Last Name English Meaning A Special Glossary Close Ad A Dictionary of German Aachen/Aix-la-Chapelle Names Aachen/Achen (German city) Hans Bahlow, Edda ... Best Price $16.95 Abend/Abendroth evening/dusk or Buy New $19.90 Abt abbott Privacy Information Ackerman(n) farmer Adler eagle Amsel blackbird B Finding Your German Ancestors Bach brook Kevan M Hansen Best Price $4.40 Bachmeier farmer by the brook or Buy New $9.13 Bader/Baader bath, spa keeper Privacy Information Baecker/Becker baker Baer/Bar bear Barth beard Bauer farmer, peasant In Search of Your German Roots. -

Family Group Sheets Surname Index
PASSAIC COUNTY HISTORICAL SOCIETY FAMILY GROUP SHEETS SURNAME INDEX This collection of 660 folders contains over 50,000 family group sheets of families that resided in Passaic and Bergen Counties. These sheets were prepared by volunteers using the Societies various collections of church, ceme tery and bible records as well as city directo ries, county history books, newspaper abstracts and the Mattie Bowman manuscript collection. Example of a typical Family Group Sheet from the collection. PASSAIC COUNTY HISTORICAL SOCIETY FAMILY GROUP SHEETS — SURNAME INDEX A Aldous Anderson Arndt Aartse Aldrich Anderton Arnot Abbott Alenson Andolina Aronsohn Abeel Alesbrook Andreasen Arquhart Abel Alesso Andrews Arrayo Aber Alexander Andriesse (see Anderson) Arrowsmith Abers Alexandra Andruss Arthur Abildgaard Alfano Angell Arthurs Abraham Alje (see Alyea) Anger Aruesman Abrams Aljea (see Alyea) Angland Asbell Abrash Alji (see Alyea) Angle Ash Ack Allabough Anglehart Ashbee Acker Allee Anglin Ashbey Ackerman Allen Angotti Ashe Ackerson Allenan Angus Ashfield Ackert Aller Annan Ashley Acton Allerman Anners Ashman Adair Allibone Anness Ashton Adams Alliegro Annin Ashworth Adamson Allington Anson Asper Adcroft Alliot Anthony Aspinwall Addy Allison Anton Astin Adelman Allman Antoniou Astley Adolf Allmen Apel Astwood Adrian Allyton Appel Atchison Aesben Almgren Apple Ateroft Agar Almond Applebee Atha Ager Alois Applegate Atherly Agnew Alpart Appleton Atherson Ahnert Alper Apsley Atherton Aiken Alsheimer Arbuthnot Atkins Aikman Alterman Archbold Atkinson Aimone -

Surname Folders.Pdf
SURNAME Where Filed Aaron Filed under "A" Misc folder Andrick Abdon Filed under "A" Misc folder Angeny Abel Anger Filed under "A" Misc folder Aberts Angst Filed under "A" Misc folder Abram Angstadt Achey Ankrum Filed under "A" Misc folder Acker Anns Ackerman Annveg Filed under “A” Misc folder Adair Ansel Adam Anspach Adams Anthony Addleman Appenzeller Ader Filed under "A" Misc folder Apple/Appel Adkins Filed under "A" Misc folder Applebach Aduddell Filed under “A” Misc folder Appleman Aeder Appler Ainsworth Apps/Upps Filed under "A" Misc folder Aitken Filed under "A" Misc folder Apt Filed under "A" Misc folder Akers Filed under "A" Misc folder Arbogast Filed under "A" Misc folder Albaugh Filed under "A" Misc folder Archer Filed under "A" Misc folder Alberson Filed under “A” Misc folder Arment Albert Armentrout Albight/Albrecht Armistead Alcorn Armitradinge Alden Filed under "A" Misc folder Armour Alderfer Armstrong Alexander Arndt Alger Arnold Allebach Arnsberger Filed under "A" Misc folder Alleman Arrel Filed under "A" Misc folder Allen Arritt/Erret Filed under “A” Misc folder Allender Filed under "A" Misc folder Aschliman/Ashelman Allgyer Ash Filed under “A” Misc folder Allison Ashenfelter Filed under "A" Misc folder Allumbaugh Filed under "A" Misc folder Ashoff Alspach Asper Filed under "A" Misc folder Alstadt Aspinwall Filed under "A" Misc folder Alt Aston Filed under "A" Misc folder Alter Atiyeh Filed under "A" Misc folder Althaus Atkins Filed under "A" Misc folder Altland Atkinson Alwine Atticks Amalong Atwell Amborn Filed under -
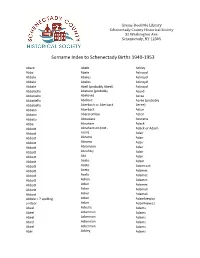
Surname Index to Schenectady Births 1940-1953
Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Abare Abele Ackley Abba Abele Ackroyd Abbale Abeles Ackroyd Abbale Abeles Ackroyd Abbale Abell (probably Abeel) Ackroyd Abbatiello Abelone (probably Acord Abbatiello Abelove) Acree Abbatiello Abelove Acree (probably Abbatiello Aberbach or Aberback Aeree) Abbato Aberback Acton Abbato Abercrombie Acton Abbato Aboudara Acucena Abbe Abraham Adack Abbott Abrahamson (not - Adack or Adach Abbott nson) Adair Abbott Abrams Adair Abbott Abrams Adair Abbott Abramson Adair Abbott Abrofsky Adair Abbott Abt Adair Abbott Aceto Adam Abbott Aceto Adamczak Abbott Aceto Adamec Abbott Aceto Adamec Abbott Acken Adamec Abbott Acker Adamec Abbott Acker Adamek Abbott Acker Adamek Abbzle = ? spelling Acker Adamkiewicz unclear Acker Adamkiewicz Abeel Ackerle Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abel Ackley Adams Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Adams Adamson Ahl Adams Adanti Ahles Adams Addis Ahman Adams Ademec or Adamec Ahnert Adams Adinolfi Ahren Adams Adinolfi Ahren Adams Adinolfi Ahrendtsen Adams Adinolfi Ahrendtsen Adams Adkins Ahrens Adams Adkins Ahrens Adams Adriance Ahrens Adams Adsit Aiken Adams Aeree Aiken Adams Aernecke Ailes = ? Adams Agans Ainsworth Adams Agans Aker (or Aeher = ?) Adams Aganz (Agans ?) Akers Adams Agare or Abare = ? Akerson Adams Agat Akin Adams Agat Akins Adams Agen Akins Adams Aggen Akland Adams Aggen Albanese Adams Aggen Alberding Adams Aggen Albert Adams Agnew Albert Adams Agnew Albert or Alberti Adams Agnew Alberti Adams Agostara Alberti Adams Agostara (not Agostra) Alberts Adamski Agree Albig Adamski Ahave ? = totally Albig Adamson unclear Albohm Adamson Ahern Albohm Adamson Ahl Albohm (not Albolm) Adamson Ahl Albrezzi Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. -

Geographic Names
GEOGRAPHIC NAMES CORRECT ORTHOGRAPHY OF GEOGRAPHIC NAMES ? REVISED TO JANUARY, 1911 WASHINGTON GOVERNMENT PRINTING OFFICE 1911 PREPARED FOR USE IN THE GOVERNMENT PRINTING OFFICE BY THE UNITED STATES GEOGRAPHIC BOARD WASHINGTON, D. C, JANUARY, 1911 ) CORRECT ORTHOGRAPHY OF GEOGRAPHIC NAMES. The following list of geographic names includes all decisions on spelling rendered by the United States Geographic Board to and including December 7, 1910. Adopted forms are shown by bold-face type, rejected forms by italic, and revisions of previous decisions by an asterisk (*). Aalplaus ; see Alplaus. Acoma; township, McLeod County, Minn. Abagadasset; point, Kennebec River, Saga- (Not Aconia.) dahoc County, Me. (Not Abagadusset. AQores ; see Azores. Abatan; river, southwest part of Bohol, Acquasco; see Aquaseo. discharging into Maribojoc Bay. (Not Acquia; see Aquia. Abalan nor Abalon.) Acworth; railroad station and town, Cobb Aberjona; river, IVIiddlesex County, Mass. County, Ga. (Not Ackworth.) (Not Abbajona.) Adam; island, Chesapeake Bay, Dorchester Abino; point, in Canada, near east end of County, Md. (Not Adam's nor Adams.) Lake Erie. (Not Abineau nor Albino.) Adams; creek, Chatham County, Ga. (Not Aboite; railroad station, Allen County, Adams's.) Ind. (Not Aboit.) Adams; township. Warren County, Ind. AJjoo-shehr ; see Bushire. (Not J. Q. Adams.) Abookeer; AhouJcir; see Abukir. Adam's Creek; see Cunningham. Ahou Hamad; see Abu Hamed. Adams Fall; ledge in New Haven Harbor, Fall.) Abram ; creek in Grant and Mineral Coun- Conn. (Not Adam's ties, W. Va. (Not Abraham.) Adel; see Somali. Abram; see Shimmo. Adelina; town, Calvert County, Md. (Not Abruad ; see Riad. Adalina.) Absaroka; range of mountains in and near Aderhold; ferry over Chattahoochee River, Yellowstone National Park. -

Curriculum Vitae
Curriculum Vitae Personal Information Name: Joshua Morrison Smyth Address: Department of Biobehavioral Health 231 Biobehavioral Health Building Pennsylvania State University University Park, PA 16802, USA Phone: (814) 863-8402 Electronic mail: [email protected] [email protected] Website: sites.psu.edu/shadelab/ Education Ph.D. Health and Social Psychology, concentration in Quantitative Methods, Stony Brook University (1998). Advisor: Arthur Stone M.A. Psychology, Stony Brook University (1994) B.A. Cognitive Science, Vassar College (1991) Professional Experience 2017- Distinguished Professor of Biobehavioral Health and Medicine, Pennsylvania State University and Hershey Medical Center 2015-2017 Academic Director, Survey Research Center, Pennsylvania State University 2015 Acting Co-Director, Clinical and Translational Sciences Institute [CTSI], Pennsylvania State University (01/01/15-06/30/15) 2014- Associate Director, Social Science Research Institute, Pennsylvania State University (Acting Director, 01/01/15-06/30/15) 2014- Associate Director, Children Youth and Family Consortium, Pennsylvania State University (Acting Director, 01/01/15-06/30/15) 2012- Faculty Affiliate, Methodology Center, Pennsylvania State University 2011-2017 Professor of Biobehavioral Health and Medicine, Pennsylvania State University and Hershey Medical Center 2011-2014 Founding Academic Director, Dynamic Real-time Ecological Ambulatory Methodologies [DREAM] Initiative, Pennsylvania State University 2009-2011 Trustee Professor, Department of Psychology, Syracuse University -

Iron, Steel and Swords Script - Page 1 Steel Steel, with Plenty of Slag Inclusions
10.3.2 The Iron Trade The town I grew up in after 1950 had around 1000 inhabitants. It was essentially a "sleeping town" for the workers and employes of the factories and businesses in the near-by cities but also still a community of farmers and artisans. Of course there was black smith (and a cartwright, glazier, carpenter, ...), and he was working right across the street from my parent's house. Same thing in all the other towns with a farming background. My grandfather actually was the black smith in another small town with less than 1000 inhabitants. So was his father and grandfather. I'm absolutely sure that any community with more than 500 inhabitants or so supported and needed a black smith ("Schmied" in German) for about 2000 years until - roughly - 1960. The surname Schmid / Schmidt / Schmitt / Schmit is No. 2 on the list of the most frequent surnames in Germany, only bested by "Müller" (you guessed it). It is clear that all those smiths' could not make their own iron / steel but bought it. The major source was the next local smelter "facility" of course, but some long-distance trade with special merchandise also existed. As soon as people started to make iron in quantities, they also started to trade the stuff. The roots of the iron trade thus go back to distant antiquity; we just don't know all that much about it. As we have learned in the preceding parts, a smelter makes a bloom, and this bloom should be processed immediately into one or several pieces of iron that can then be used for making products. -

Surname First Name Categorisation Abadin Jose Luis Silver Abbelen
2018 DRIVERS' CATEGORISATION LIST Updated on 09/07/2018 Drivers in red : revised categorisation Drivers in blue : new categorisation Surname First name Categorisation Abadin Jose Luis Silver Abbelen Klaus Bronze Abbott Hunter Silver Abbott James Silver Abe Kenji Bronze Abelli Julien Silver Abergel Gabriele Bronze Abkhazava Shota Bronze Abra Richard Silver Abreu Attila Gold Abril Vincent Gold Abt Christian Silver Abt Daniel Gold Accary Thomas Silver Acosta Hinojosa Julio Sebastian Silver Adam Jonathan Platinum Adams Rudi Bronze Adorf Dirk Silver Aeberhard Juerg Silver Afanasiev Sergei Silver Agostini Riccardo Gold Aguas Rui Gold Ahlin-Kottulinsky Mikaela Silver Ahrabian Darius Bronze Ajlani Karim Bronze Akata Emin Bronze Aksenov Stanislas Silver Al Faisal Abdulaziz Silver Al Harthy Ahmad Silver Al Masaood Humaid Bronze Al Qubaisi Khaled Bronze Al-Azhari Karim Bronze Alberico Neil Silver Albers Christijan Platinum Albert Michael Silver Albuquerque Filipe Platinum Alder Brian Silver Aleshin Mikhail Platinum Alesi Giuliano Silver Alessi Diego Silver Alexander Iradj Silver Alfaisal Saud Bronze Alguersuari Jaime Platinum Allegretta Vincent Silver Alleman Cyndie Silver Allemann Daniel Bronze Allen James Silver Allgàuer Egon Bronze Allison Austin Bronze Allmendinger AJ Gold Allos Manhal Bronze Almehairi Saeed Silver Almond Michael Silver Almudhaf Khaled Bronze Alon Robert Silver Alonso Fernando Platinum Altenburg Jeff Bronze Altevogt Peter Bronze Al-Thani Abdulrahman Silver Altoè Giacomo Silver Aluko Kolawole Bronze Alvarez Juan Cruz Silver Alzen