Neural Activity Associated with Monitoring the Oscillating Threat Value of a Tarantula
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tarantulas and Social Spiders
Tarantulas and Social Spiders: A Tale of Sex and Silk by Jonathan Bull BSc (Hons) MSc ICL Thesis Presented to the Institute of Biology of The University of Nottingham in Partial Fulfilment of the Requirements for the Degree of Doctor of Philosophy The University of Nottingham May 2012 DEDICATION To my parents… …because they both said to dedicate it to the other… I dedicate it to both ii ACKNOWLEDGEMENTS First and foremost I would like to thank my supervisor Dr Sara Goodacre for her guidance and support. I am also hugely endebted to Dr Keith Spriggs who became my mentor in the field of RNA and without whom my understanding of the field would have been but a fraction of what it is now. Particular thanks go to Professor John Brookfield, an expert in the field of biological statistics and data retrieval. Likewise with Dr Susan Liddell for her proteomics assistance, a truly remarkable individual on par with Professor Brookfield in being able to simplify even the most complex techniques and analyses. Finally, I would really like to thank Janet Beccaloni for her time and resources at the Natural History Museum, London, permitting me access to the collections therein; ten years on and still a delight. Finally, amongst the greats, Alexander ‘Sasha’ Kondrashov… a true inspiration. I would also like to express my gratitude to those who, although may not have directly contributed, should not be forgotten due to their continued assistance and considerate nature: Dr Chris Wade (five straight hours of help was not uncommon!), Sue Buxton (direct to my bench creepy crawlies), Sheila Keeble (ventures and cleans where others dare not), Alice Young (read/checked my thesis and overcame her arachnophobia!) and all those in the Centre for Biomolecular Sciences. -
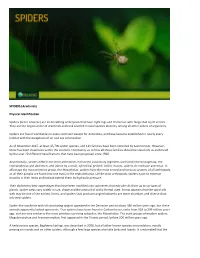
Arachnids) Physical Identification Spiders (Order Araneae
SPIDERS (Arachnids) Physical Identification Spiders (order Araneae) are air-breathing arthropods that have eight legs and chelicerae with fangs that inject venom. They are the largest order of arachnids and rank seventh in total species diversity among all other orders of organisms. Spiders are found worldwide on every continent except for Antarctica, and have become established in nearly every habitat with the exceptions of air and sea colonization. As of November 2015, at least 45,700 spider species, and 113 families have been recorded by taxonomists. However, there has been dissension within the scientific community as to how all these families should be classified, as evidenced by the over 20 different classifications that have been proposed since 1900. Anatomically, spiders differ from other arthropods in that the usual body segments are fused into two tagmata, the cephalothorax and abdomen, and joined by a small, cylindrical pedicel. Unlike insects, spiders do not have antennae. In all except the most primitive group, the Mesothelae, spiders have the most centralized nervous systems of all arthropods, as all their ganglia are fused into one mass in the cephalothorax. Unlike most arthropods, spiders have no extensor muscles in their limbs and instead extend them by hydraulic pressure. Their abdomens bear appendages that have been modified into spinnerets that extrude silk from up to six types of glands. Spider webs vary widely in size, shape and the amount of sticky thread used. It now appears that the spiral orb web may be one of the earliest forms, and spiders that produce tangled cobwebs are more abundant and diverse than orb-web spiders. -

Common Kansas Spiders
A Pocket Guide to Common Kansas Spiders By Hank Guarisco Photos by Hank Guarisco Funded by Westar Energy Green Team, American Arachnological Society and the Chickadee Checkoff Published by the Friends of the Great Plains Nature Center i Table of Contents Introduction • 2 Arachnophobia • 3 Spider Anatomy • 4 House Spiders • 5 Hunting Spiders • 5 Venomous Spiders • 6-7 Spider Webs • 8-9 Other Arachnids • 9-12 Species accounts • 13 Texas Brown Tarantula • 14 Brown Recluse • 15 Northern Black Widow • 16 Southern & Western Black Widows • 17-18 Woodlouse Spider • 19 Truncated Cellar Spider • 20 Elongated Cellar Spider • 21 Common Cellar Spider • 22 Checkered Cobweb Weaver • 23 Quasi-social Cobweb Spider • 24 Carolina Wolf Spider • 25 Striped Wolf Spider • 26 Dotted Wolf Spider • 27 Western Lance Spider • 28 Common Nurseryweb Spider • 29 Tufted Nurseryweb Spider • 30 Giant Fishing Spider • 31 Six-spotted Fishing Spider • 32 Garden Ghost Spider Cover Photo: Cherokee Star-bellied Orbweaver ii Eastern Funnelweb Spider • 33 Eastern and Western Parson Spiders • 34 Garden Ghost Spider • 35 Bark Crab Spider • 36 Prairie Crab Spider • 37 Texas Crab Spider • 38 Black-banded Crab Spider • 39 Ridge-faced Flower Spider • 40 Striped Lynx Spider • 41 Black-banded Common and Convict Zebra Spiders • 42 Crab Spider Dimorphic Jumping Spider • 43 Bold Jumping Spider • 44 Apache Jumping Spider • 45 Prairie Jumping Spider • 46 Emerald Jumping Spider • 47 Bark Jumping Spider • 48 Puritan Pirate Spider • 49 Eastern and Four-lined Pirate Spiders • 50 Orchard Spider • 51 Castleback Orbweaver • 52 Triangulate Orbweaver • 53 Common & Cherokee Star-bellied Orbweavers • 54 Black & Yellow Garden Spider • 55 Banded Garden Spider • 56 Marbled Orbweaver • 57 Eastern Arboreal Orbweaver • 58 Western Arboreal Orbweaver • 59 Furrow Orbweaver • 60 Eastern Labyrinth Orbweaver • 61 Giant Long-jawed Orbweaver • 62 Silver Long-jawed Orbweaver • 63 Bowl and Doily Spider • 64 Filmy Dome Spider • 66 References • 67 Pocket Guides • 68-69 1 Introduction This is a guide to the most common spiders found in Kansas. -

Spiders: Spooky Or Cool? Student Booklet
Spiders: Spooky or Cool? Student Booklet Texas AgriLife Extension Part of the Texas A&M University System Jeffery K Tomberlin, Heidi Myers Molly Keck, M.S. Ph.D Department of Animal Extension Program Associate Professor Science Specialist 2475 TAMU Tarleton State University 3355 Cherry Ridge, Ste. College Station, Texas 77845 Stephenville, Texas 76401 212 Email: San Antonio, Texas 78230 [email protected] Email: [email protected] Preface Spiders make up the largest group of arachnids. Despite the handful of poisonous species, many spiders are harmless contrary to fears and beliefs. Although the mere presence of spiders and their webs can be a nuisance, they are beneficial because they prefer to feed on harmful insects (such as flies) and mites. Spiders are often called insects or “bugs,” but they are actually quite different and the distinction between the two and among common species should be known. Some examples of different types of spiders include: the black widow, brown recluse, sac spiders, tarantulas, jumping spiders, wolf spiders, and orbweavers. In this booklet are a variety of exercises designed to help you learn more about spiders and way they may be beneficial or harmful. 1 Tables of Contents Preface Page 1 Lesson 1 – What is a Spider? Page 3 Activity 1-1 – Spiders vs. Insects Page 6 Activity 1-2 – Arthropod Body Parts Matching Game Page 8 Lesson 2 – Beneficial and Harmful Spiders Page 10 Activity 2-1 – Harmful and Beneficial Spiders Page 13 Activity 2-2 – Harmful and Beneficial Spiders Matching Game Page 14 Lesson -

Past Life Regression
Past Life Regression Past life regression is a technique that uses hypnosis to recover the memories of past lives or incarnations. Past-life regression is typically undertaken either in pursuit of a spiritual experience, or in a psychotherapeutic setting. Most advocates loosely adhere to beliefs about reincarnation, though religious traditions that incorporate reincarnation generally do not include the idea of repressed memories of past lives. The technique used during past-life regression involves the subject answering a series of questions while hypnotized to reveal identity and events of their past lives. The use of hypnosis and suggestive questions can tend to help the subject to recall his past memories. The source of the memories is more likely that of combine experiences, knowledge, imagination and suggestion or guidance from the hypnotist or regression therapist. Once created, those memories are indistinguishable from memories based on events that occurred during the subject's life. Experiments with subjects undergoing past-life regression indicate that a belief in reincarnation and suggestions by the hypnotist are the two most important factors regarding the contents of memories reported. In the 2nd century BC, Sage Patanjali, in his Yoga Sutras, discussed the idea of the soul becoming burdened with an accumulation of impressions as part of the karma from previous lives. Patanjali called the process of past-life regression “prati- prasav”or "reverse birthing" and saw it as addressing current problems through memories of past lives. Past life regression can be found in Jainism. The seven truths of Jainism deal with the soul and its attachment to karma and Moksha. -
Types of Phobias Can Disrupt the Regular Functioning of a Person, There Are Effective Treatments for Which You Must Reach out to the Psychoterapist
INTRODUCING Top 10 Commo n Phobias Phobias are psychological disorder defined by persistent fear of object or situations. 1. MYSOPHOBIA It is a pathological fear of contamination and germs and also usually relates with OCD. Victims keep washing their hands to protect themselves from germs and even own a collection of disinfectants. 2. ARACHNOPHOBIA The fear of Spiders and other arachnids denote this kind of phobia. In fact, the picture of spider alone is enough for the panic. Such people avoid the places where they think spiders and arachnids could be found. 3. ACROPHOBIA The fear of heights may lead to anxiety attack among more than 6% of acrophibics. Acrophobics avoid going to tall buildings, mountains or tall towers in fear of falling down. 4. OPHIDIOPHOBIA Ophidiophobia is one of the most common phobias and is related to fear of snakes. The disgust feeling by looking at snakes or snake pictures may appear because of personal experiences or cultural influence. 5. AEROPHOBIA As the name suggests, the fear of flying is called as Aerophobia. Though the plane accidents are uncommon but this fear lingers in approximately 10%-40% U.S. adults.. Rapid heartbeat and trembling is common with this type of phobia. 6. CYNOPHOBIA Cynophobia is a type pf phobia where a person is afraid of dogs and this fear is mostly associated with personal experiences. Such person keep a safe distance from the streets or houses where dogs wander and live. Top 10 Common Phobias "Phobias are treatable!" Phobias are psychological disorder defined by persistent fear of object or situations. -

Media Representation of Spiders May Exacerbate Arachnophobic Sentiments By
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.28.065607; this version posted April 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Media representation of spiders may exacerbate arachnophobic sentiments by 2 framing a distorted perception of risk 3 4 Stefano Mammola1, *, Veronica Nanni2, Paolo Pantini3, Marco Isaia4 7 1 Molecular Ecology Group (MEG), Water Research Institute, National Research Council of Italy (CNR-IRSA), 8 Largo Tonolli 50, 28922 Verbania Pallanza, Italy 9 2 Department of Earth, Environmental and Life Science (DISTAV), University of Genoa, Via Balbi, 5, 16126 10 Genova, Italy 11 3 Museo civico di Scienze Naturali “E. Caffi.”, Piazza Cittadella 10, 24129, Bergamo, Italy 12 4 Department of Life Sciences and Systems Biology, University of Turin, Via Accademia Albertina 13, 10123 13 Torino, Italy 14 16 *corresponding author: [email protected] 17 (Orcid ID: http://orcid.org/0000-0002-4471-9055) 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.04.28.065607; this version posted April 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 18 ABSTRACT 19 Spiders are able to arouse strong emotional reactions in humans. While spider bites are 20 statistically rare events, our perception is skewed towards the potential harm spiders can cause to 21 humans. -

Spider World Records: a Resource for Using Organismal Biology As a Hook for Science Learning
A peer-reviewed version of this preprint was published in PeerJ on 31 October 2017. View the peer-reviewed version (peerj.com/articles/3972), which is the preferred citable publication unless you specifically need to cite this preprint. Mammola S, Michalik P, Hebets EA, Isaia M. 2017. Record breaking achievements by spiders and the scientists who study them. PeerJ 5:e3972 https://doi.org/10.7717/peerj.3972 Spider World Records: a resource for using organismal biology as a hook for science learning Stefano Mammola Corresp., 1, 2 , Peter Michalik 3 , Eileen A Hebets 4, 5 , Marco Isaia Corresp. 2, 6 1 Department of Life Sciences and Systems Biology, University of Turin, Italy 2 IUCN SSC Spider & Scorpion Specialist Group, Torino, Italy 3 Zoologisches Institut und Museum, Ernst-Moritz-Arndt Universität Greifswald, Greifswald, Germany 4 Division of Invertebrate Zoology, American Museum of Natural History, New York, USA 5 School of Biological Sciences, University of Nebraska - Lincoln, Lincoln, United States 6 Department of Life Sciences and Systems Biology, University of Turin, Torino, Italy Corresponding Authors: Stefano Mammola, Marco Isaia Email address: [email protected], [email protected] The public reputation of spiders is that they are deadly poisonous, brown and nondescript, and hairy and ugly. There are tales describing how they lay eggs in human skin, frequent toilet seats in airports, and crawl into your mouth when you are sleeping. Misinformation about spiders in the popular media and on the World Wide Web is rampant, leading to distorted perceptions and negative feelings about spiders. Despite these negative feelings, however, spiders offer intrigue and mystery and can be used to effectively engage even arachnophobic individuals. -

Brown Recluses (Loxosceles Reclusa) (Fig Goods &-Om out of State
Medical Myth f\~yth: idiopathic wounds are often due to brovvn recluse or other spider bites throughout the United States INTRODUCTION mens were individuals intercepted from facilities receiving Richard S Vetter Spiders, especially brown recluses (Loxosceles reclusa) (fig goods &-om out of state. There are no populations of Department of ure 1), are frequently incriminated as causative agents in brown recluses in California. Entomology idiopathic wounds, even though diagnoses are usually University of California Riverside, CA 92521 made solely on the basis of dermatologic symptoms. MISDIAGNOSIS OF IDIOPATHIC WOUNDS Rarely is a spider seen inflicting the wound or captured Correspondence to: Despite the lack of brown recluse spiders in California, while biting, and it is difficult to either prove or disprove Dr Vetter several hundred cases of "brown recluse bites" have been spider participation in the event. The biologic distribution [email protected] reported to me in the past decade. Undoubtedly, this is of the brown recluse and related recluse species indicates only a small fraction of the total number of brown recluse Competing interests: that many diagnoses made on cases occurring in the west bites that have been diagnosed. Although it might appear None dedared ern United States are incorrect.1 intuitive from figure 2 that these bites could caused by be West]Med the native desert recluse (Loxosceles deserta), which lives in 2000;173:357-358 SOURCES OF INFORMATION the comparatively sparsely inhabited southeastern quad Because of my research interests in spider identification rant of California, virtually all so-called brown recluse bites and medically important spiders, I have been avidly study have come from coastal regions with a concentrated hu ing recluse spiders and their occurrence in the western man population. -

Growing up with Arachnophobia: Helping Adults Through Outreach
Growing Up With Arachnophobia: Helping Adults Through Outreach Winnifred Wolfe Introduction I conducted this study because I wanted to know more about the root cause of adult arachnophobia. I was hoping to find out more about where and why the fear started so I could try to help adults overcome fear. This research has taught me a lot about arachnophobia because I now know common reactions adults have near spiders. The reason I focused on working with adults is because as people grow up their fears start to grow as well whereas when people are little they do not know to fear spiders. It is my hope that through my results I can learn more about adults reactions to spiders and can help them face and overcome their fear. Literature Review I looked through various articles about helping people with arachnophobia. Bouchard et al said that using virtual reality helps people with their fears. This article also said that arachnophobia was the most common complaint. The treatment in the “Effectiveness of Virtual Reality”study helps recreate situations virtually that would be hard to remake in real life and can also let the therapist watch the participant interact with their fear. Eleven people did this treatment, ten females and one male. All of the patients’ phobia started when they were little. They did five sessions a week for ninety minutes. These patients were all eighteen and older. No huge difference was noted after the patients went through the treatment. Later, Shahriari-Namadi et al discussed how females are more likely to experience phobic disorder than males. -

The Wonder Weekly October 12, 2020
The Published by the Peter Underwood Centre October 12, 2020 Follow us on Facebook The ships Solve the without word a crew: changer: Page 2 www.facebook.com/ Page 2 UnderwoodCentre/ ZOOPHOBIAS Solve the tangled web of animal phobias Ailurophobia Musophobia Ophidiophobia Arachnophobia Cynophobia Redback spiders prey mainly on insects, but also catch small lizards and occasionally snakes in their sticky webs. Share projects inspired by The Wonder Weekly with us. Email: [email protected] Picture: iStock/ Wrennie ARE there animals that frighten know with a certain amount of people who suffer from it and is relating to animals. Broadly Your challenge is to match the you? caution. often the result of a bad these are called zoophobias, but phobias to the animal or class of In many instances it is a good But some people have a fear of experience. there are also individual phobia animals they relate to (e.g. arachnophobia is the fear of idea to be careful. all dogs, are frightened when You are probably aware that names for the fear of spiders, arachnids, such as spiders and dogs are around and try to avoid there are many different phobias. snakes, sharks and so on. For example, you might be scorpions). perfectly comfortable around them at all times. For example, you might have How many do you think you Children’s University Tasmania could name? your family’s pet dog. This fear of dogs is called heard of acrophobia, which is a members can earn stamps in However, even though most cynophobia. fear of heights. -

An Introduction to Common of Sri Lanka
www.dilmahconservation.org An introduction to common of Sri Lanka SpidersAuthored by Ranil P. Nanayakkara 1 Declaration of Our Core Commitment to Sustainability Dilmah owes its success to the quality of Ceylon Tea. Our business was founded therefore on an enduring connection to the land and the communities in which we operate. We have pioneered a comprehensive commitment to minimising our impact on the planet, fostering respect for the environment and ensuring its protection by encouraging a harmonious coexistence of man and nature. We believe that conservation is ultimately about people and the future of the human race, that efforts in conservation have associated human well-being and poverty reduction outcomes. These core values allow us to meet and exceed our customers’ expectations of sustainability. Our Commitment We reinforce our commitment to the principle of making business a matter of human service and to the core values of Dilmah, which are embodied in the Six Pillars of Dilmah. We will strive to conduct our activities in accordance with the highest standards of corporate best practice and in compliance with all applicable local and international regulatory requirements and conventions. We recognise that conservation of the environment is an extension of our founding commitment to human service. We will assess and monitor the quality and environmental impact of its operations, services and products whilst striving to include its supply chain partners and customers, where relevant and to the extent possible. We are committed to transparency and open communication about our environmental and social practices. We promote the same transparency and open communication from our partners and customers.