Attribution Enhanced: an SEM/EDS - LA-ICP-MS Investigation of a Suspected Mid-18Th-Century Wistarburgh Glass Globular Bottle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

C:\Documents and Settings\Owner
A Multi-Media Guide to Shopping, Dining, Lodging, Recreation, Entertainment, Art & Historic Points of Interest for The American Heritage Tourist EVENTS... 4 INDEX OF CITIES... 6-7 ON THE ROAD... 27 Establish Your Community as a GREAT AMERICAN DESTINATION FALL / WINTER 2017-2018 (for less than a dime a day per lister) www.AmericanAntiquities.com See inside front cover 2 / AMERICAN JOURNAL Volume 25, FALL/WINTER 2017-18 AMERICAN ANTIQUITIES JOURNAL Volume 25, FALL/WINTER 2017-18/ 3 Enjoy your next road trip to one of our 500+ client cities. Let this be your guide for AboutAbout thethe CoCovverer shopping, dining, lodging, recreation, entertainment & historic points of interest for the AMERICAN HERITAGE TOURIST www.AmericanAntiquities.com Depression glass is clear or a specified number of colored translucent magazine subscriptions, thus glassware. It was produced in making its way into almost a multitude of colors, ranging every American home. from the deep colors of purple, Depression glass started one black, cobalt, and red to the of the largest collecting trends pastels of pink, yellow, green, ever, from collectors to amber, and blue which nostalgia hunters. Over created to bring a bright note 100,000 eager collectors now into the otherwise drab times seek this prized glass, of the depression. Most of this whether to complete a glassware was made in the handed-down family set of Ohio River Valley of the United dishes, or to find the highly States, where access to raw sought and elusive rare materials and power made pieces. manufacturing inexpensive. Depression glass is More than twenty becoming more scarce on the manufacturers made more open market. -

XIX Century Murano Glass Tableware Kindle
GLASS FOR THE TABLE : XIX CENTURY MURANO GLASS TABLEWARE PDF, EPUB, EBOOK Andrea Morucchio | 51 pages | 01 Jan 2000 | Arsenale | 9788877432131 | English, Italian | San Giovanni Lupatoto, United States Glass for the Table : XIX Century Murano Glass Tableware PDF Book Sort By:. Item Location see all. Fusing The process of founding or melting the batch. Heating pieces of glass in a kiln or furnace until they bond. The unofficial capital of glassmaking is perhaps known best for their dazzling chandeliers and opulent vases, which add a pop of color and sophistication to any room. Mentioned for accuracy Sign In Register. Cart 0. Originally established in in Pittsburgh , the first city to use coal for fuel in glassmaking, the company survived under several different firms until Much modern glass must be heated to about 2, degrees Farenheit, followed by a maturing period when the molten glass cools to a working temperature of about 2, degrees Farenheit. For a downloadable PDF, click here 27 pages. Since childhood he has shown great interest in art. Attributed to Salviati Dott. After annealing, the disk is cut into panes. In the master suite of a Long Island home, a motorized cabinet containing a Samsung television stands at the foot of the bed. Result is a polychrome design that is visible only when seen in cross section. So called because one surviving example is said to have belonged to Saint Hedwig of Silesia. Made by inflating a large gather, swinging it until it forms a cylinder, detaching it from the blowpipe, cutting it lengthwise, reheating it, and allowing it to slump to the form of a flat sheet. -

Pennsylvania Folklife Vol. 29, No. 2 Ivy Kemp Yost
Ursinus College Digital Commons @ Ursinus College Pennsylvania Folklife Magazine Pennsylvania Folklife Society Collection Winter 1980 Pennsylvania Folklife Vol. 29, No. 2 Ivy Kemp Yost John D. Kendig William Munro Howell J. Heaney Carter W. Craigie See next page for additional authors Follow this and additional works at: https://digitalcommons.ursinus.edu/pafolklifemag Part of the American Art and Architecture Commons, American Material Culture Commons, Christian Denominations and Sects Commons, Cultural History Commons, Ethnic Studies Commons, Fiber, Textile, and Weaving Arts Commons, Folklore Commons, Genealogy Commons, German Language and Literature Commons, Historic Preservation and Conservation Commons, History of Religion Commons, Linguistics Commons, and the Social and Cultural Anthropology Commons Click here to let us know how access to this document benefits oy u. Recommended Citation Yost, Ivy Kemp; Kendig, John D.; Munro, William; Heaney, Howell J.; Craigie, Carter W.; and Twiss, Beth Ann, "Pennsylvania Folklife Vol. 29, No. 2" (1980). Pennsylvania Folklife Magazine. 87. https://digitalcommons.ursinus.edu/pafolklifemag/87 This Book is brought to you for free and open access by the Pennsylvania Folklife Society Collection at Digital Commons @ Ursinus College. It has been accepted for inclusion in Pennsylvania Folklife Magazine by an authorized administrator of Digital Commons @ Ursinus College. For more information, please contact [email protected]. Authors Ivy Kemp Yost, John D. Kendig, William Munro, Howell J. Heaney, Carter W. Craigie, and Beth Ann Twiss This book is available at Digital Commons @ Ursinus College: https://digitalcommons.ursinus.edu/pafolklifemag/87 ~ontril1utor~ . IVY KEMP YOST has written a number of accounts of the local history of Eastern Berks County. -

Facilities on the RCRA GPRA Cleanup Baseline
Facilities on the RCRA GPRA Cleanup Baseline Most of these facilities were identified in the early 1990's when EPA and the states were prioritizing their corrective action workload, and were identified as facilities where early cleanup progress would be appropriate. Today, many of these facilities have already made substantial progress in their cleanup efforts. Some of these facilities have met the environmental indicator (EI) measures and at some of these facilities cleanup is complete. Many of the facilities that have not yet met the EI measures have still made substantial progress by stabilizing the problems or in some cases beginning final remedies. Some percentage of these facilities have not yet been assessed by EPA or the states for EI determinations. When assessed, it may be determined that some of these facilities currently meet EI measures. EPA will be focusing resources on meeting the environmental indicators for the facilities on this Baseline. The company names found on this list are the current facility owners. There may be companies on this baseline that are the current property owner but did not cause the contamination. There may also be cases where the former owners have entered an agreement to be responsible for cleanup. Unfortunately, the database is unable to track and identify these instances. Human Exposure Environmental Indicator Groundwater Environmental Indicator CA725 (Status Code) CA750 (Status Code) YE - "Current Human Exposures Under Control" has YE - Yes, "Migration of Contaminated Groundwater been verified. Under Control" has been verified. NO - "Current Human Exposures" are NOT "Under NO - Unacceptable migration of contaminated Control." groundwater is observed or expected. -

Lamps, Maps, Mud-Machines, and Signal Flags: Science, Technology, and Commerce in the Early United States James Russell Risk University of South Carolina
University of South Carolina Scholar Commons Theses and Dissertations 2017 Lamps, Maps, Mud-Machines, and Signal Flags: Science, Technology, and Commerce in the Early United States James Russell Risk University of South Carolina Follow this and additional works at: https://scholarcommons.sc.edu/etd Part of the History Commons Recommended Citation Risk, J. R.(2017). Lamps, Maps, Mud-Machines, and Signal Flags: Science, Technology, and Commerce in the Early United States. (Doctoral dissertation). Retrieved from https://scholarcommons.sc.edu/etd/4203 This Open Access Dissertation is brought to you by Scholar Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. LAMPS, MAPS, MUD-MACHINES, AND SIGNAL FLAGS: SCIENCE, TECHNOLOGY, AND COMMERCE IN THE EARLY UNITED STATES by James Russell Risk Bachelor of Arts Fairmont State University, 2008 Master of Arts University of Maryland Baltimore County, 2011 Submitted in Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy in History College of Arts and Sciences University of South Carolina 2017 Accepted by: Allison C. Marsh, Major Professor Carol E. Harrison, Committee Member Cary J. Mock, Committee Member Joseph A. November, Committee Member Cheryl L. Addy, Vice Provost and Dean of the Graduate School © Copyright by James Russell Risk, 2017 All Rights Reserved. ii DEDICATION To Ann Johnson (1965-2016): mentor, colleague, and friend. iii ACKNOWLEDGEMENTS I would like to acknowledge my original advisor, the accomplished and adept Ann Johnson, who passed away December 11, 2016. Ann’s expertise and knowledge were an invaluable resource. -

The Bible in Iron
;:,! LIBKARY UNIVERSITY^ PENN5YL\^\NIA T39.4 nS3S GIFT OF Dr. (S-HORGE E NITZSCHE i ^Phe Bible in Iron OR The Pictured Stoves and Stove Plates of the Pennsylvania Germans BY HENRY C. MERCER Published for the Bucks County Historical Society Doylestown, Pennsylvania EA\9KIALL!BRARY^.sPvBUCArS3 iF THE X^NIVERSITT^*- PENNSYL^/ANIAJ ACCE-SSISN ^^ PRESeiNTED BY , PREFACE. The art of making iron stoves decorated with pictures and designs illustra- ting the teachings of the Bible was brought from Germany to the Anglo-Ameri- can colonies and survived for half a century on American soil. Cast in low relief upon the flat and often polished sides of the old stoves, continually confronting the settler and his family at one of the centres of house- hold comfort in winter, the singular patterns, generally explained with inscrip- tions, telling of the Miracles of Christ and the Prophets, the beauty of Holiness, and the lessons of Vice and Virtue must have impressed many minds. But the effort of the writer has been rather to explain and describe the casters' art which then, as an inheritance from Germany, gradually appeared and suddenly ceased, than to account for the influence of its teaching upon the lives of the colonists and their descendants. Yet. within the last few weeks, a new and unexpected significance has attached itself to these iron pictures, once so full of meaning in the pioneer household, so long forgotten and now at last rescued from the dust of ruins. For now 'October, 1914,) in the midst of the great European conflict, the ancestral land which made them is passing through an hour of trial. -
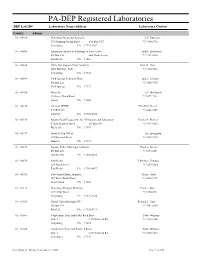
PA-DEP Registered Laboratories DEP Lab ID# Laboratory Name/Address Laboratory Contact
PA-DEP Registered Laboratories DEP Lab ID# Laboratory Name/Address Laboratory Contact County: Adams 01- 00046 Gettysburg Municipal Authority Jeff Patterson 576 Pumping Station Road P O Box 3307 717-334-6738 Gettysburg PA 17325-3307 01- 00550 Laboratory Analytical & Biological Services Inc Judith Ziolkowski PO Box 836 409 North Avenue 717-259-6550 East Berlin PA 17316 01- 00634 White Run Regional Mun Authority John W Deitz 2001 Baltimore Park 717-334-7476 Gettysburg PA 17325 01- 00699 York Springs Treatment Plant James Lehman PO Box 222 717-528-7955 York Springs PA 17372 01- 00704 Motts Inc Jeff McDannell 45 Aspers North Road 717-677-7121 Aspers PA 17304 01- 00710 Orrtanna WWTF Harold N Kessel P O Box 526 717-642-8509 Fairfield PA 17320-0526 01- 00715 Knouse Foods Cooperative Inc - Environmental Laboratory Charles A Bennett 53 East Hanover Street PO Box 807 717-677-9115 Biglerville PA 17307 01- 00717 Berwick Twp WWTF Jay Livingston 85 Municipal Road 717-624-2712 Hanover PA 17331 01- 00855 Possum Valley Municipal Authority Thomas Beamer PO Box 420 717-677-8551 Bendersville PA 17306-0420 01- 00870 East Berlin T Michael Thoman 128 Water Street 717-259-9224 East Berlin PA 17316-8637 01- 00986 New Oxford Muni Authority Barry Groft 409 Water Works Road 717-624-9399 New Oxford PA 17350 01- 01142 Gettysburg Hospital Wellspan Charles Kyle 147 Gettys Street 717-334-2121 Gettysburg PA 17325-2534 01- 01639 Carroll Valley Borough STP Richard L Cool PO Box 718 717-642-8269 Fairfield PA 17320-0718 01- 01685 Cumberland Twp Auth-Table Rock Road Todd Williams -
Facilities on the RCRA GPRA Cleanup Baseline
Facilities on the RCRA GPRA Cleanup Baseline Most of these facilities were identified in the early 1990's when EPA and the states were prioritizing their corrective action workload, and were identified as facilities where early cleanup progress would be appropriate. Today, many of these facilities have already made substantial progress in their cleanup efforts. Some of these facilities have met the environmental indicator (EI) measures and at some of these facilities cleanup is complete. Many of the facilities that have not yet met the EI measures have still made substantial progress by stabilizing the problems or in some cases beginning final remedies. Some percentage of these facilities have not yet been assessed by EPA or the states for EI determinations. When assessed, it may be determined that some of these facilities currently meet EI measures. EPA will be focusing resources on meeting the environmental indicators for the facilities on this Baseline. The company names found on this list are the current facility owners. There may be companies on this baseline that are the current property owner but did not cause the contamination. There may also be cases where the former owners have entered an agreement to be responsible for cleanup. Unfortunately, the database is unable to track and identify these instances. Human Exposure Environmental Indicator Groundwater Environmental Indicator CA725 (Status Code) CA750 (Status Code) YE - "Current Human Exposures Under Control" has YE - Yes, "Migration of Contaminated Groundwater been verified. Under Control" has been verified. NO - "Current Human Exposures" are NOT "Under NO - Unacceptable migration of contaminated Control." groundwater is observed or expected. -

The Stiegel/Old Wedding Goblet
OUR HERITAGE www.manheim1762.org 717-665-5560 Published by the Historic Manheim Preservation Foundation, Inc., 27 Market Square, Manheim, PA 17545 April 2017 THE STIEGEL OLD WEDDING GOBLET On March 23, 1773, we assume he put his financial troubles behind him and joined in the celebration of the marriage of his daughter, Elizabeth, to William Old. The March 29, 1773, issue of Pennsylvania Chronicle carried the following announcement: "On Tuesday, the 23rd ult was married at Manheim, in county of Lancaster, Mr. William Old, junior of Speedwell forge, ironmaster to Miss Elizabeth Stiegel, eldest daughter of Mr. Henry William Stiegel, proprie- tor of the American Flint Glass Manufactory." We are fortunate that young Mr. Old picked this particular time to declare his love for Elizabeth Stiegel. If he had waited another year or two his fa- HMPF received permission from The National ther-in-law would not have been able to present the Early American Glass Club, www.glassclub.org, to newlyweds with the engraved wedding goblet which reprint this article by Sheldon D. Butts, which ap- is subject of this article. The Old Wedding Goblet has peared in the Winter 1983 issue of The Glass Club been passed down in the family for over two hundred years and is now available for study by both scholars Bulletin. and collectors. This goblet is important because it ——— holds the promise of being the first piece of glass that In 1773 Henry William Stiegel was both at the can be confidently attributed to Henry Stiegel’s height of his success and on the brink of financial American Flint Glass Manufactory. -

Ladies' Home Journal 1898
™. _ A1MECDQTAL SIDE QF MRS- CLEVELAND IN THIS NUMBER JUNE, 1898 MRS. GROVER CLEVELAND, FROM HER LATEST PORTRAIT, TAKEN AT PRINCETON, NEW JERSEY, MARCH, 1898, WITH THE HOME OF MR. AND MRS. CLEVELAND IN THE BACKGROUND «\ it THE CURTIS PUBLISHING COMPANY, PHILADELPHIA THE CENTRAL NEWS COMPANY, PHILADELPHIA, GENERAL AGENTS "Mary, how do you find the Ivory Soap does?" " Best we have ever had, Ma'am. The starched clothes are whiter and the flannels are softer than when washed with the soaps we have been using. It saves my hands, ***** Long ago distanced all competitors/' — Medical Standard. Ma'am ; they used to be very sore after a wash." PACKER'S TAR SOAP " Well, Mary, I intend to have you use only Ivory Soap has exceptional value in summer. It quickly relieves the after this, for I am told that it saves the clothes, too." - irritations caused by Prickly Heat, Chafing, Perspiration, Stings, Ivy Poisoning, etc.; is delightfully refreshing for Those who have tried both common soap and Ivory Soap Bath and Shampoo, and wards off Contagion. say that it takes only two-thirds as muck of the Ivory for a wash. THE PACKER MFO. CO., 81 FULTON STREET, NEW YORK Copyright, loUS. b, The PrMIrr * Oarobli Co., ClMUMatl. We will send you THE SATURDAY EVENING POST every week from now to January i, 1899- the balance of this year-on receipt of only 25 cents (stamps or silver). The regular subscription price is $2.50 per year. A WEEKLY MAGAZINE HANDSOMELY ILLUSTRATED Founded A. D. i?28 by Benjamin Franklin. ,6 pages without an uninteresting paragraph. -

Lancaster Mennonite Historical Society's 202D Auction of Rare, Out-Of-Print, and Used Books Friday, December 12, 2003, at 6:30
LANCASTER MENNONITE HISTORICAL SOCIETY’S 202D AUCTION OF RARE, OUT-OF-PRINT, AND USED BOOKS FRIDAY, DECEMBER 12, 2003, AT 6:30 P.M. TEL: (717) 393-9745; FAX: (717) 393-8751; EMAIL: [email protected] WEBSITE: http://www.lmhs.org/ Please click here for book auction policies and procedures before bidding . Click here for a key to abbreviations used in this catalog . The Lancaster Mennonite Historical Society will conduct its 202d auction on December 12, 2003, at 2215 Millstream Road, Lancaster, Pennsylvania, one-half mile east of the intersection of Routes 30 and 462. This will be the final sale for 2003. The auction not only specializes in local and denominational history and genealogy of southeastern Pennsylvania, but also includes theological works and other types of material of interest to the nationwide constituency. Please note the changes for 2004: Sale dates for 2004 are as follows: March 12, June 11, and September 10. There may be a fourth auction on December 10 if needed. Individual print catalogs are available from the Society for $8.00 ($4.00 for Society members) + $3.00 postage and handling. Persons who wish to be added to the mailing list for 2004 may do so by sending $24.00 ($12.00 for Society members) with name and address to the Society. Higher rates apply for subscribers outside of the United States. All subscriptions expire at the end of the calendar year. 1. Baker, N.B. Early Houses of New England , 1967. 143+pp 11.25” (b/w ill, dj clipped, vgc); Davidson, M.B. -

Books on Glass - Joslin Hall Rare Books - Catalog 346 Books on Glass - Joslin Hall Rare Books - Catalog 346
BOOKS ON GLASS - JOSLIN HALL RARE BOOKS - CATALOG 346 BOOKS ON GLASS - JOSLIN HALL RARE BOOKS - CATALOG 346 Joslin Hall Rare Books Post Office Box 239 Northampton, Mass 01061 telephone: (413) 247-5080 e-mail: [email protected] website: www.joslinhall.com Member- Antiquarian Booksellers Association of America International League of Antiquarian Booksellers -Telephone reservations are highly recommended. -Standard courtesies are extended to institutions and dealers. -Postage charges are additional. -We are happy to arrange lay-away terms to fit your needs. -All books may be returned within ten days of receipt -please notify us in advance and repack the book/s carefully in the original box (if possible); please make sure that the parcel is properly insured. Checks, American Express, Discover,Visa, Mastercard & Paypal accepted. BOOKS ON GLASS - JOSLIN HALL RARE BOOKS - CATALOG 346 1. American Silver and Pressed Glass. A Collection in the R.W. Norton Art Gallery. Shreveport; R.W. Norton Art Foundation: 1967. A catalog produced for a special exhibition which coincided with the 1967 annual 'Holiday in Dixie' celebrations in Shreveport, which commemorate the Louisiana purchase in 1803. The silver includes pieces made between 1695 and 1797 in Boston, New York and Philadelphia. The pressed glass is made up of examples of the Lion and Westward Ho pattern, both produced by Philadelphia's Gillinder & Sons factory in the 1870s. The text includes essays on both glass patterns. The museum had only opened in 1966, and this was the first catalog featuring objects from its permanent collection. Softcover. 8.5"x11", 68 pages, b/w illustrations.